
Concept explainers
(a)
Interpretation: The conformation which is present in higher concentration when
Concept introduction: The change in Gibbs free energy is represented by
If the
Answer to Problem 6.37P
The equatorial conformation is present in higher concentration in the given compound.
Explanation of Solution
Given:
The value of
The equilibrium reaction of monosubstituted cyclohexane is shown below.
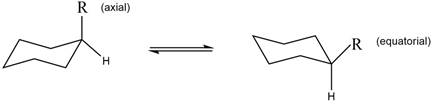
Figure 1
The value of
The equatorial conformation is present in higher concentration in the given compound.
(b)
Interpretation: The
Concept introduction: The change in Gibbs free energy is represented by
If the
Answer to Problem 6.37P
The
Explanation of Solution
The values of
The both given values are greater than
If the
Therefore, the
The
(c)
Interpretation: The
Concept introduction: The change in Gibbs free energy is represented by
If the
Answer to Problem 6.37P
The
Explanation of Solution
The values of
The equilibrium constant
Therefore, the
The
(d)
Interpretation: The
Concept introduction: The change in Gibbs free energy is represented by
If the
Answer to Problem 6.37P
The value of
Explanation of Solution
Given:
The values of
The relationship between
As the value of
The value of
(e)
Interpretation: The explanation corresponding to the relation of size of
Concept introduction: The change in Gibbs free energy is represented by
If the
Answer to Problem 6.37P
The large size of
Explanation of Solution
The value
The large size of
Want to see more full solutions like this?
Chapter 6 Solutions
KCTCS Organic Chemistry Value Edition (Looseleaf) - Text Only
- Draw the skeletal (bond-line) structures of all isomers of C4H8O (including configurational isomers) that contain an alkene and an ether. There should be 5 structures.arrow_forwardWrite the balanced chemical equation for the complete combustion of ethane. Reactants Products C2H6 (g) + → Formulate (skeletal formula) the main products for the oxidation and dehydration reactions of the following compound: Reactant Oxidation product Dehydration products | OH CH3―CH2―CH―CH2―CH3arrow_forwardRank the conformations of n-butane with reference to its potential energy from the most stable to the least stable. Rank from the most stable to the least stable. To rank items as equivalent, overlap them.arrow_forward
- Rank the conformations of n-butane with reference to its potential energy from the most stable to the least stable. Rank from the most stable to the least stable.To rank items as equivalent, overlap them.arrow_forwardIf a catalyst could be found that would establish an equilibrium between 1,2-butadiene and 2-butyne, what would be the ratio of the more stable isomer to the less stable isomer at 25°C? CH,=C=CHCH, CH,C=CCH, AG° = -16.7 kJ (-4.0 kcal)/molarrow_forwardViewed through the C-3 — C-4 bond, which is the most stable conformation of 3-bromo-2-methylpentane?arrow_forward
- TRUE OR FALSE (a) Both ethylene and acetylene are planar molecules. (b) An alkene in which each carbon of the double bond has two different groups bonded to it will show cis-trans isomerism. (c) Cis-trans isomers have the same molecular formula but a different connectivity of their atoms. (d) Cis-2-butene and trans -2-butene can be interconverted by rotation about the carbon–carbon double bond. (e) Cis-trans isomerism is possible only among appropriately substituted alkenes. (f) Both 2-hexene and 3-hexene can exist as pairs of cis-trans isomers. (g) Cyclohexene can exist as a pair of cis-trans isomers. (h) 1-Chloropropene can exist as a pair of cis-trans isomers.arrow_forwardWhich of the following cycloalkanes are capable of geometric (cis-trans) isomerism?Draw the cis and trans isomers.(a) 3-ethyl-1,1-dimethylcyclohexane (b) 1-ethyl-3-methylcycloheptane(c) 1-ethyl-3-methylcyclopentane (d) 1-cyclopropyl-2-methylcyclohexanearrow_forwardFor trans‑1‑ethyl‑4‑isopropylcyclohexane, which structures represent the possible boat conformations?arrow_forward
- 2,3-Dibromoprop-1-ene (C3H4Br2) has four H atoms. Suppose that any of these H atoms can be replaced by a Cl atom to yield a molecule with the formula C3H3Br,Cl. (a) Identify two H atoms where this substitution would yield constitutional isomers of C3H3Br,CI; (b) enantiomers of C3H3Br,Cl; (c) diastereomers of C3H3Br,Cl. H нн H Br Br 2,3-Dibromoprop-1-enearrow_forwardCyclopropane (C3H6, a three-membered ring) is more reactive than most other cycloalkanes.(a) Draw a Lewis structure for cyclopropane.(b) Compare the bond angles of the carbon atoms in cyclopropane with those in an acyclic (noncyclic) alkane.(c) Suggest why cyclopropane is so reactive.arrow_forwardAs we learned in Chapter 4, monosubstituted cyclohexanes exist as an equilibrium mixture of two conformations having either an axial or equatorial substituent. When R = CH2CH3, Keq for this process is 23.When R = C(CH3)3, Keq for this process is 4000. a.When R = CH2CH3, which conformation is present in higher concentration? b.Which R shows the higher percentage of equatorial conformation at equilibrium? c. Which R shows the higher percentage of axial conformation at equilibrium? d. For which R is ΔGo more negative? e.How is the size of R related to the amount of axial and equatorial conformations at equilibrium?arrow_forward
 Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning


