
Concept explainers
(a)
Interpretation:
The number and kind of stereoisomers formed when given molecule is treated in presence of
Concept Introduction:
Geometric isomers: Two atoms with the same side location of the double bond are called cis isomers and two atoms with opposite side locations of the double bond are called Trans isomers.
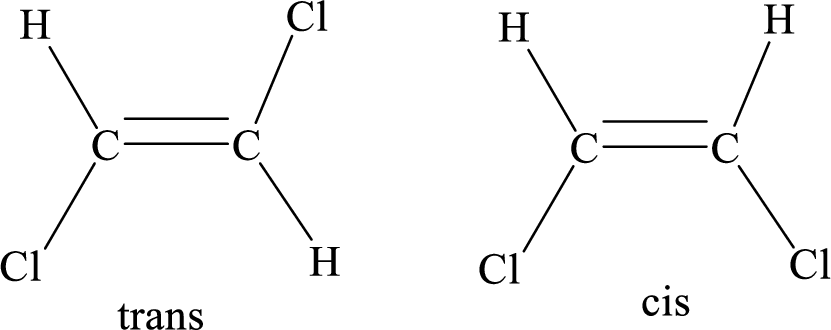
R and S nomenclature: it is used to assign the molecule using CIP (Cahn-Ingold-Prelog) rules.
The CIP rules are as follows:
Select the chiral carbon and assign the numbers according to the decreasing
If the numbering follows clockwise direction then the molecule is termed as R and if it follows anti-clockwise direction then molecule is termed as S.
(b)
Interpretation:
The number and kind of stereoisomers formed when given molecule is treated in presence of
Concept Introduction:
Geometric isomers: Two atoms with the same side location of the double bond are called cis isomers and two atoms with opposite side locations of the double bond are called Trans isomers.
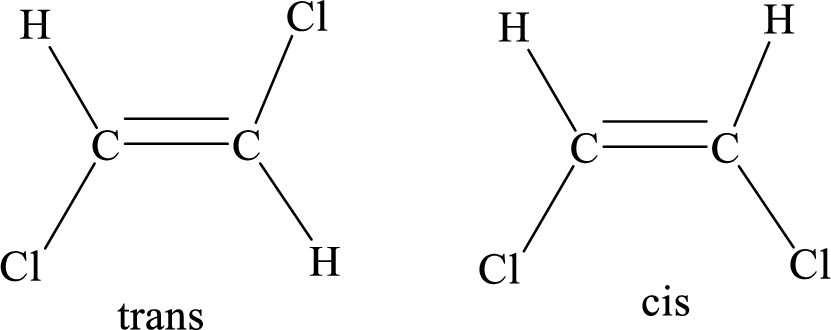
R and S nomenclature: it is used to assign the molecule using CIP (Cahn-Ingold-Prelog) rules.
The CIP rules are as follows:
Select the chiral carbon and assign the numbers according to the decreasing atomic mass of atoms attached to it.
If the numbering follows clockwise direction then the molecule is termed as R and if it follows anti-clockwise direction then molecule is termed as S.
(c)
Interpretation:
The number and kind of stereoisomers formed when given molecule is treated in presence of
Concept Introduction:
Geometric isomers: Two atoms with the same side location of the double bond are called cis isomers and two atoms with opposite side locations of the double bond are called Trans isomers.
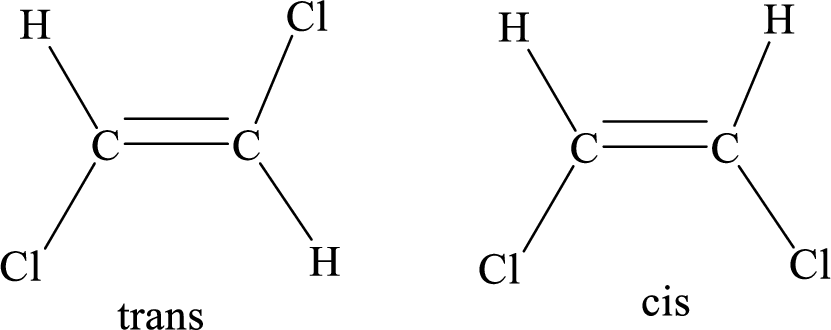
R and S nomenclature: it is used to assign the molecule using CIP (Cahn-Ingold-Prelog) rules.
The CIP rules are as follows:
Select the chiral carbon and assign the numbers according to the decreasing atomic mass of atoms attached to it.
If the numbering follows clockwise direction then the molecule is termed as R and if it follows anti-clockwise direction then molecule is termed as S.
(d)
Interpretation:
The number and kind of stereoisomers formed when given molecule is treated in presence of
Concept Introduction:
Geometric isomers: Two atoms with the same side location of the double bond are called cis isomers and two atoms with opposite side locations of the double bond are called Trans isomers.
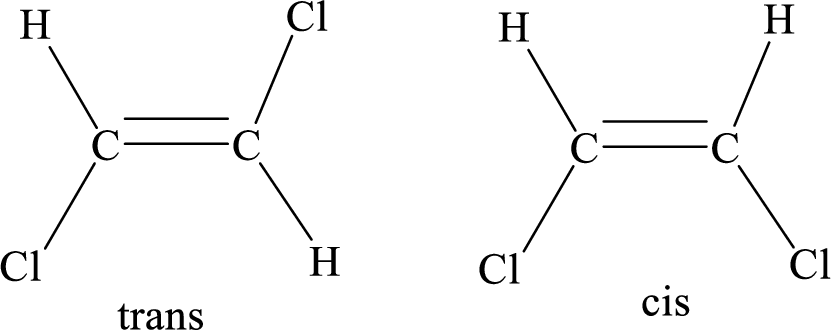
R and S nomenclature: it is used to assign the molecule using CIP (Cahn-Ingold-Prelog) rules.
The CIP rules are as follows:
Select the chiral carbon and assign the numbers according to the decreasing atomic mass of atoms attached to it.
If the numbering follows clockwise direction then the molecule is termed as R and if it follows anti-clockwise direction then molecule is termed as S.
Trending nowThis is a popular solution!

Chapter 6 Solutions
Organic Chemistry
- Write the structure of the compound E,E-2,4-Hexadien-1-ol and label each non-equivalent carbon with a letter, A,B,C..arrow_forwardAn achiral hydrocarbon A of molecular formula C7H12 reacts with two equivalents of H2 in the presence of Pd-C to form CH3CH2CH2CH2CH(CH3)2. One oxidative cleavage product formed by the treatment of A with O3 is CH3COOH. Reaction of A with H2 and Lindlar catalyst forms B, and reaction of A with Na, NH3 forms C. Identify compounds A, B, and C. Be sure to answer all parts.arrow_forwardWhich of the following alkene reactions occurs with a complete cleavage of the C=C ( breaking both pi and sigma bond components) ? Select one: A. Alkene + H2O2 with catalytic amount of OsO4 B. Reaction of an alkene with KMnO4/OH- at room temperature. C. Treating the alkene with O3 gas, followed by reduction with Zn / H2O D. Reaction of an alkene with water (H2O) in the presence of a H+ catalyst.arrow_forward
- Draw the products formed when both cis- and trans-but-2-ene are treated with OsO4, followed by hydrolysis with NaHSO3 + H2O. Explain how these reactions illustrate that syn dihydroxylation is stereospecific.arrow_forwardDraw the products formed when both cis- and trans-but-2-ene are treated with a peroxyacid followed by -OH (in H2O).Explain how these reactions illustrate that anti dihydroxylation is stereospecic.arrow_forwardDraw the products formed when both cis- and trans-but-2-ene are treated with OsO4, followed by hydrolysis with NaHSO3 + H2O. Explain how these reactions illustrate that syn dihydroxylation is stereospecic.arrow_forward
- Draw the constitutional isomer formed when the following alkenes are treated with each set of reagents: [1] H2O, H2SO4; or [2] BH3 followed by H2O2, −OH.arrow_forwardDraw the structure of two alkenes that would yield 1‑methylcyclohexanol when treated with Hg(OAc)2Hg(OAc)2 in water, then NaBH4NaBH4arrow_forwardAlcohol A (C10H18O) is converted to a mixture of alkenes B and C on being heated with potassium hydrogen sulfate (KHSO4). Catalytic hydrogenation of B and C yields the same product. Assuming that dehydration of alcohol A proceeds without rearrangement, deduce the structures of alcohol A and alkene C.arrow_forward
- Compound A (C11H23Br) is a secondary alkyl halide. On being heated with a solution of sodium ethoxide in ethanol, compound A yielded a mixture of two alkenes B and C, each having molecular formula C11H22. Catalytic hydrogenation of the major isomer B or the minor isomer C gave only 3,5-diethylheptane. Draw structures for compounds A, B, and C consistent with these observations.arrow_forwardDraw the organic products formed when cyclopentene is treated with each reagent. With some reagents, no reactionoccurs.a. H2 + Pd-Cb. H2 + Lindlar catalystc. Na, NH3d. CH3CO3He. [1] CH3CO3H; [2] H2O, HO–f. [1] OsO4 + NMO; [2] NaHSO3, H2Og. KMnO4, H2O, HO–h. [1] LiAlH4; [2] H2Oi. [1] O3; [2] CH3SCH3j. (CH3)3COOH, Ti[OCH(CH3)2]4, (–)-DETk. mCPBAl. Product in (k); then [1] LiAlH4; [2] H2Oarrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning

