
Concept explainers
The chapter sections to review are shown in parentheses at the end of each problem.
Using the models of the molecules (black = C, white = H, yellow = S, green = Cl), determine each of the following for models of compounds 1 and 2: (7.2, 7.4, 7.5, 7.6)
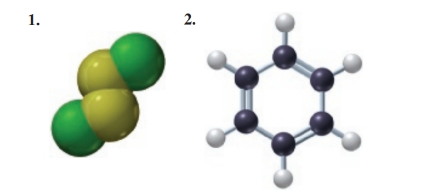
a. molecular formula
c. molar mass
b. empirical formula
d. mass percent composition
(a)
Interpretation: To find the empirical formula of dipyrithione.
Concept Introduction: Empirical formula is the simplest ratio of different elements in a compound.
Answer to Problem 61UTC
The empirical formula of dipyrithione is C5H4NOS
Explanation of Solution
The molecular formula of dipyrithione is C10H8N2O2S2 The subscript of each element is divided by 2. 2 is the minimum number of atoms of elements N, O and S.
Thus the empirical formula is C5H4NOS
(b)
Interpretation: To find the molar mass of dipyrithione.
Concept Introduction: Molar mass is found by adding the atomic mass of each element that makes up the compound.
Answer to Problem 61UTC
Molar mass of dipyrithione = 252 g/mol
Explanation of Solution
The molecular formula of dipyrithione is C10H8N2O2S2 Atomic mass of C = 12 g/mol
Atomic mass of H = 1 g/mol
Atomic mass of N = 14 g/mol
Atomic mass of O = 16 g/mol
Atomic mass of S = 32 g/mol
Molar mass of dipyrithione, C10H8N2O2S2 =
(c)
Interpretation: To find the mass percent of O in dipyrithione.
Concept Introduction:
Answer to Problem 61UTC
Mass percent of O in dipyrithione = 12.7 %
Explanation of Solution
The molecular formula of dipyrithione is C10H8N2O2S2 Since from the molecular formula of dipyrithione it’s clear that dipyrithione contains 2 atoms of oxygen so, Mass percent of O in dipyrithione =
(d)
Interpretation: To find the grams of C in 2.50 g of dipyrithione.
Concept Introduction: An arithmetical multiplier which is used for converting a quantity expressed in one unit into another equivalent set of units is said to be conversion factor.
Answer to Problem 61UTC
11.9 g of C is present in 2.50 g of dipyrithione.
Explanation of Solution
The molecular formula of dipyrithione is C10H8N2O2S2 Since from the molecular formula of dipyrithione it’s clear that dipyrithione contains 10 atoms of carbon so,
Thus,
Hence, the mass of carbon in 25.0 g of dipyrithione is 11.9 g.
(e)
Interpretation: To find the moles of dipyrithione in 25.0 g of dipyrithione.
Concept Introduction:
The relationship between mass, moles, and molar mass:
Answer to Problem 61UTC
Moles of dipyrithione = 0.0992 mol
Explanation of Solution
The relationship between mass, moles, and molar mass:
Mass of dipyrithione is 25.0 g (given)
Molar mass of dipyrithione is 252 g/mol.
Moles of dipyrithione =
Want to see more full solutions like this?
Chapter 7 Solutions
Basic Chemistry
- 14. Romeo preserves a dozen of milkfish for his birthday celebration. He used 200g of NaCl to fully preserve it in just 3 days. How many moles of NaCl do the 200g have? A. 1. 42 mole NaCl B. 2. 42 mole NaCl C. 3. 42 mole NaCl D. 4. 42 mole NaClarrow_forwarda cereal contains 11 grams of sucrose per 60g of cereal. How many grams of cereal must be eated to consume 0.0424 moles of sucrosearrow_forwardInitial Temp of HCl: 22.96 C Final Temp of HCl: 28.30 C Moles of HCl: .05 moles Moles of NaOH: .05 moles Cs water: 4.184 J/g C Accepted delta Hrxn: -55.8 kJ/mol 1. what is the qwater. pls include formula. 2. calculate delta Hrxnn using the moles, temp and cswater 3. calculate delta Hrxn % errorarrow_forward
- Give answer to all parts? Initial Temp of HCl: 22.96 C Final Temp of HCl: 28.30 C Moles of HCl: .05 moles Moles of NaOH: .05 moles Cs water: 4.184 J/g C Accepted delta Hrxn: -55.8 kJ/mol 1. what is the qwater. pls include formula. 2. calculate delta Hrxnn using the moles, temp and cswater 3. calculate delta Hrxn % errorarrow_forwardThe AP Biology teacher is measuring out 647.0 g of dextrose (C6H12O6) for a lab. How many moles of dextrose is this equivalent to?arrow_forwarda hydrated CuSO4 salt was heated and thus dehydrated. Determine the % H2O by weight in the hydrated CuSO4 salt. SHOW WORK TO RECEIVE FULL CREDIT mass of empty dish 43.571 gmass of dish + hydrated salt 44.326 gmass of dish + dehydrated salt 44.054 g 4. Using the data found in question number 3, determine the empirical formula for the hydrated CuSO4salt. What is the chemical name of this dehydrated salt? 5. Suppose that after your second heating, you were impatient and did not wait until your porcelain evaporating dish to cool. Instead, you weighed it while it was still hot. How would this specifically affect the calculated percentage of water in the compound?arrow_forward
- A student reacted 3.01g salicylic acid with 5.98mL acetic anhydride and isolated 2.85g Asprin. A) Calculate moles of salicylic acid used. B) Calculate the mass of acetic anhydride used. C) Calculate moles of acetic anhydride used. D) using moles of limiting reactant present, calculate the theoretical yield of aspirin. E) Calculate the percent yield of aspirin synthesis.arrow_forwardIf the empirical formula of a compound is found to be CH2, which of the following value is a plausible molecular mass for the compound? The answer is 70.15. How did you get that?arrow_forwardCalculate the percent error for magnesium carbonate with a measured percent magnesium of 29.4%. Group of answer choices 58.0% 1.95% 36.7% 1.99% 98.1%arrow_forward
- 1. Water is formed by burning H2 (g) in the presence of O2 (g): 2H2 (g) + O2 (g) à 2H2O (g) a) If you have 32 g of H2 gas and 32 g of O2 gas, which do you have the most of in terms of moles? Explain your reasoning. b) Starting from the 32 g of H2, how many moles of water can form from the H2 gas? c) Starting from the 32 g of O2, how many moles of water can form from the O2 gas? d) Based on the amounts of water formed in parts b) and c), which is the limiting reactant for this reaction? H2 gas or O2 gas? Explain your reasoning. e) According to the limiting reactant you chose in part d), what is theoretical yield in grams of water that could be produced when 32 g of H2 gas reacts with 32 g of O2 gas?arrow_forwardA student reacted 1.01g salicylic acid with 2.06mL acetic anhydride and isolated 1.40g aspirin. A) Calculate moles of acetic anhydride used. B) Using the moles of limiting reactant present, calculate the theoretical yield of aspirin. C) Calculate the percent yield of the aspirin synthesis.arrow_forwardAmmonium nitrate fertilizer is sometimes used as an explosive. How many moles of water can be formed from the decomposition of 13.2 moles of ammonium nitrate? 2NH4NO3 ® 2N2 + O2 + 4H2Oarrow_forward
 Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
