
Concept explainers
(a)
Interpretation:
The structure and the stereochemistry of products formed by the reaction,
Concept introduction:
The addition of
Answer to Problem 7.55AP
The products formed by the reaction,
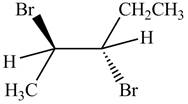
The stereo isomeric products that are formed are in the same amounts. Therefore, the racemic mixture is obtained.
Explanation of Solution
The reaction of
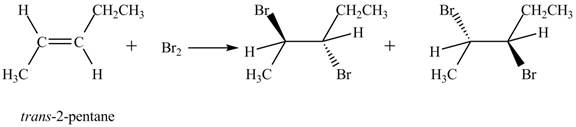
Figure 1
The products obtained are in equal ratio. Therefore, the racemic mixture is obtained.
The products formed by the reaction
(b)
Interpretation:
The structure and the stereochemistry of products formed by the reaction,
Concept introduction:
The addition of
Answer to Problem 7.55AP
The products formed by the reaction
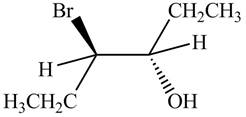
The stereo isomeric products that are formed are in the same amounts. Therefore, the racemic mixture is obtained.
Explanation of Solution
The reaction of
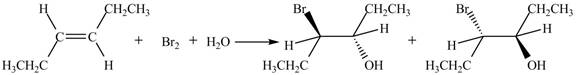
Figure 2
The products obtained are in equal ratio. Therefore, the racemic mixture is obtained.
The products formed by the reaction
(c)
Interpretation:
The structure and the stereochemistry of products formed by the reaction
Concept introduction:
The addition of
Answer to Problem 7.55AP
The products formed by the reaction
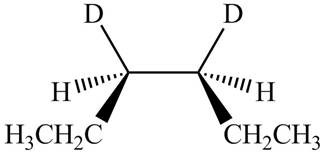
The stereoisomeric product that is obtained by the
Explanation of Solution
The reaction of
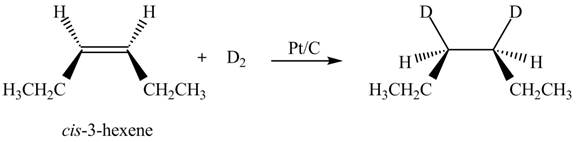
Figure 3
Therefore, the compound obtained is meso compound.
The products formed by the reaction
The stereo isomeric product that is obtained by the
(d)
Interpretation:
The structure and the stereochemistry of products formed by the reaction
Concept introduction:
The addition of
Answer to Problem 7.55AP
The product formed by the reaction,
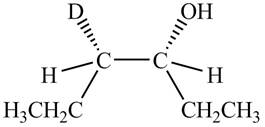
The stereo isomeric products that are formed are in the same amounts. Therefore, the racemic mixture is obtained.
Explanation of Solution
The reaction of
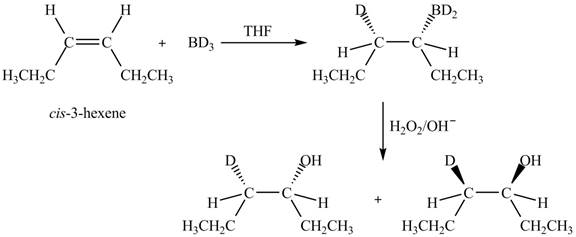
Figure 4
The products obtained are in equal ratio. Therefore, the racemic mixture is obtained.
The products formed by the reaction
The stereo isomeric products that are formed are in the same amounts.
Want to see more full solutions like this?
Chapter 7 Solutions
Organic Chemistry 6e & Study Guide
- Q3. For the following reactions, predict the products, mechanism and name all the products obtained Catalytic hydrogenation of cycloalkenes. Reduction of cyclohexanone with Zinc amalgam (Zn/Hg alloy) in concentrated hydrochloric acid Addition of CH2I2 in an alkene in the presence of Zn-Cu 2-hexene + chlorine gas Halogenation of cyclohexane in the presence of light and catalyst Sulfonation of Cyclobutane with oleum Addition of HBr to methylcyclopropanearrow_forward7. When butan-2-ol undergoes acid catalysed dehydration reaction, a mixture of products, B and C, are formed.Compound C shows cis-trans isomerism. Ozonolysis of compound B yields D and E. Draw the structure of Band C, and determine the major product. Draw cis-trans isomers of C. Write the reaction equation for theozonolysis of compound B that yields D and E. Show the preparation of butan-2-ol via a Grignard reagent byusing a suitable alkyl halide and compound D and E.arrow_forwardExplain the stereochemistry of E2 eliminations to form alkenes, and predict the products of E1 reactions on stereoisomers and on cyclohexane systems.arrow_forward
- Write out all the isomers of the compound with molecular formula C4H10O . Select the normal/ primaryisomer and treat it with conc. H2SO4 and heat. Identify the reaction and give the product ‘A’ from it. 2. When ‘A’ is treated with HBr in the presence of a peroxide, give the name and structure of the product.arrow_forwardPredict the major products of the following reactions. Include appropriate stereochemistry in the product structures. S-1,3-dimethylcyclohexene + aqueous Hg(OAc)2Hg(OAc)2 followed by treatment with NaBH4arrow_forwardCompound A has the formula C9H19Cl. B is a C9H19Br compound.A and B undergo base-promoted E2 elimination to give the same alkene C as the major product as well as different minor products.C reacts with one molar equivalent of hydrogen in the presence of a palladium catalyst to form 2,6-dimethylheptane.Addition of HCl to C yields A as the major product.Propose structures for A and B.arrow_forward
- Deduce the structure of each compound from the information given. All unknowns in this problem have molecularformula C8H12.(a) Upon catalytic hydrogenation, unknown W gives cyclooctane. Ozonolysis of W, followed by reduction with dimethylsulfide, gives octanedioic acid, HOOC¬(CH2)6¬COOH. Draw the structure of Warrow_forwardThe reaction of (S)-2-bromopentane with potassium cyanide to yield 2-methylpentanenitrile (2-cyanopentane) occurs due to a nucleophilic substitution pathway. The reaction is 100% stereospecific. Please explain what this observation tells about the mechanism of the reaction.arrow_forward(10pts) Compound A, C10H16, was found to be optically active. On catalytic reduction over a palladium catalyst, 2 equivalents of hydrogen were absorbed, yielding compound B, CioH2o. On ozonolysis of A, two fragments were obtained. One fragment was identified as acetic acid (CHCOOH). The other fragment, compound C, was an optically active carboxylic acid, C8H14O2. Write reactions, and draw the correct structures for A-C, explain your answer in detail.arrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

