
(a)
All of the constants in the equation fir tensile yield strength as function of solid solution composition.
(a)
Answer to Problem 7.6P
The constants in the equation fir tensile yield strength as function of solid solution composition is
Explanation of Solution
Concept used:
Draw the diagram for yield strength of iron as a function of the atom fraction of carbon to the one-half power as shown below:
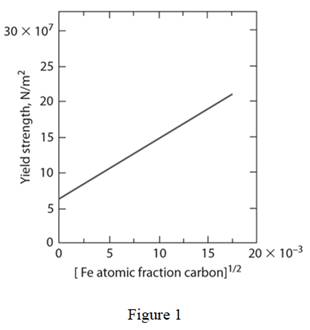
Write the expression for tensile strength of solid solution strengthened alloy.
Here,
Calculation:
Refer to figure 1; the value of
Refer to figure 1; at atom fraction of carbon as
Substitute
Conclusion:
Thus, the constants in the equation fir tensile yield strength as function of solid solution composition is
(b)
The yield strength of iron-carbon alloy.
(b)
Answer to Problem 7.6P
The yield strength of iron-carbon alloyis
Explanation of Solution
Substitute
Conclusion:
Thus, the yield strength of iron-carbon alloyis
(c)
Comment if fraction of carbon increased thenthe strength to be given by an extension of the line in the figure or not.
(c)
Explanation of Solution
If the concentration of solid solution atoms becomes large then the precipitation of atoms starts to form. As mentioned if concentration is increased from
The strength dependence of precipitates is different than the strength dependence of solid solution. Therefore, it is not possible to give extension of line in figure.
Want to see more full solutions like this?
Chapter 7 Solutions
Materials Science And Engineering Properties
 Materials Science And Engineering PropertiesCivil EngineeringISBN:9781111988609Author:Charles GilmorePublisher:Cengage Learning
Materials Science And Engineering PropertiesCivil EngineeringISBN:9781111988609Author:Charles GilmorePublisher:Cengage Learning
