
Concept explainers
Interpretation:
The structures of the major organic products formed in the reaction of
Concept introduction:
When an unsymmetrically substituted alkene reacts with a hydrogen halide, the hydrogen adds to the carbon that has the greater number of hydrogens, and the halogen adds to the carbon that has fewer hydrogens. This rule is called Markovnikov’s rule.
During hydroboration oxidation, hydrogen forms a bond with the carbon atom that has fewer hydrogens attached to it and the hydroxyl atom forms a bond with the carbon atom that has a greater number of hydrogens attached to it. This is a rule opposite to the Markovnikov’s addition.
Answer to Problem 28P
Solution:
Explanation of Solution
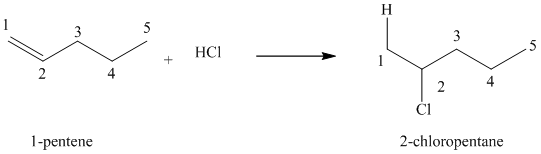
(a) Reaction of

The given alkene,
Hydrogen chloride gets added to the double bond of
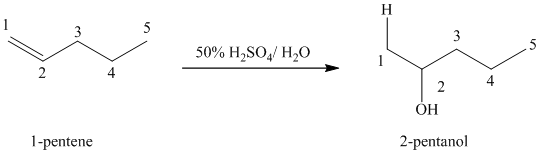
(b) Reaction of
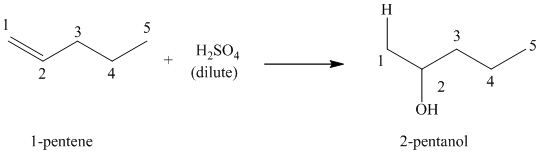
This reaction is an acid catalyzed electrophilic addition reaction of alkenes in which water molecule adds to the double bond in
A molecule of water adds to the double bond of
The addition mechanism for this reaction follows the Markovnikov’s rule. Therefore, the major organic product for the above acid-catalyzed electrophilic addition reaction is
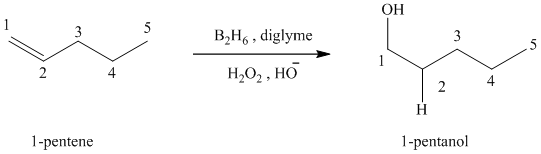
(c) Reaction of
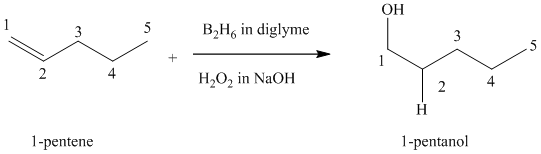
Hydroboration-oxidation leads to the overall hydration of an alkene. In hydroboration-oxidation,
The hydrogen atom in the water molecule adds to the carbon
In case of
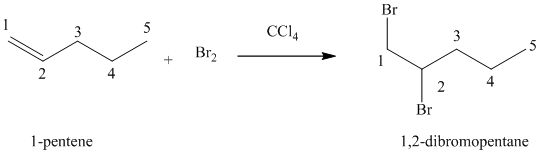
(d) Reaction of

Bromine reacts rapidly with alkenes by electrophilic addition. The products are called vicinal dibromides, meaning that the bromine atoms get attached to adjacent double bonded carbon atoms. It is carried out in suitable solvents like
A molecule of bromine adds across the double bond in
(e) Reaction of
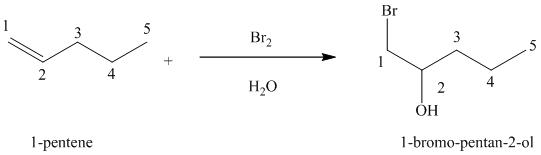
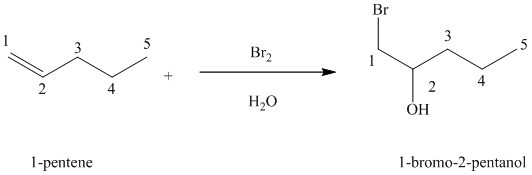
Chlorine and bromine react with alkenes in aqueous solution to give the corresponding vicinal halohydrins – compounds that add a halogen and hydroxyl group on adjacent carbon atoms in the alkene. The halogen atom forms a bond with that carbon atom in alkene, which has a greater number of hydrogen atoms, while the hydroxyl group bonds to that carbon atom in alkene, which has a fewer number of hydrogen atoms.
In the reaction of
(f) Reaction of
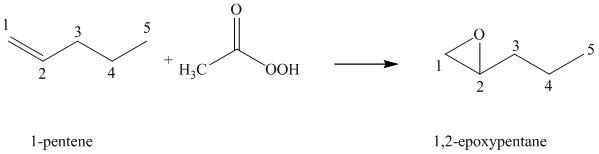
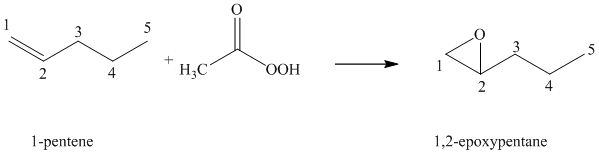
Peroxyacid transfers oxygen to the double bond of alkene to yield epoxides, which is a three-membered oxygen-containing ring.
When
(g) Reaction of
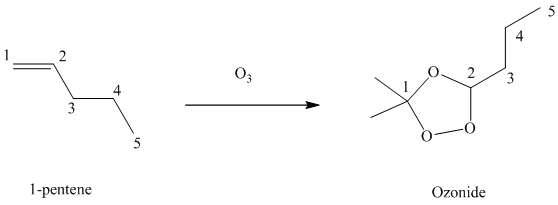
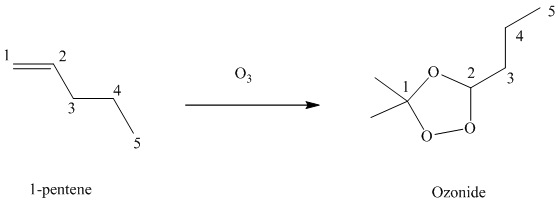
Ozone is a powerful electrophile and reacts with alkenes to cleave the double bond between two oxygen atoms in the molecule, forming an ozonide.
When
(h) Product of part (g) treated with zinc in water
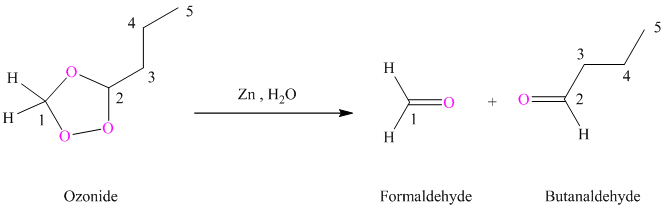
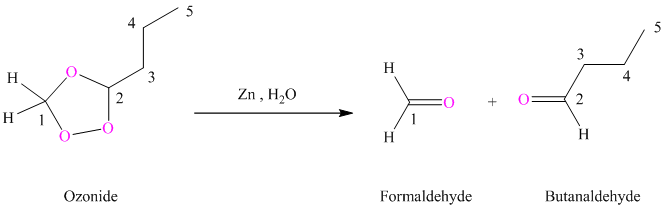
Ozonides are formed as a result of the reaction of ozone with an alkene. Ozonides undergo hydrolysis in water giving carbonyl compounds. Depending upon the structure of the starting alkene, various carbonyl compounds such as formaldehyde, aldehydes, or ketones are formed.
When corresponding ozonide of
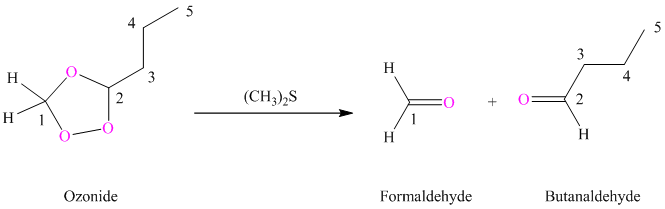
(i) Product of part (g) is treated with dimethyl sulfide.
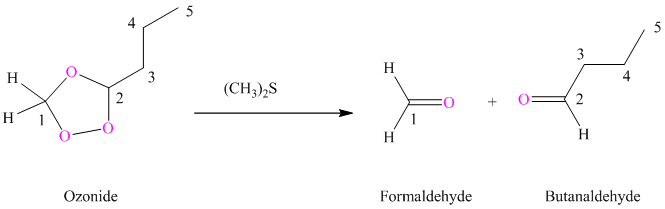
Ozonides are formed as a result of the reaction of ozone with an alkene. Ozonides undergo hydrolysis in water, giving carbonyl compounds. Depending upon the structure of the starting alkene, various carbonyl compounds such as formaldehyde, aldehydes, or ketones are formed.
When corresponding ozonide of
Want to see more full solutions like this?
Chapter 8 Solutions
ORGANIC CHEMISTRY-PACKAGE >CUSTOM<
- Compound A is an alcohol that undergoes oxidation to produce compound B.Compound B is a ketone that gives positive triiodomethane reaction. Compound B isthen reacted with phenyl magnesium bromide, C6H5MgBr in the presence of aqueousacid to form compound C. Compound C has the molecular formula of C9H12O. Deducethe structure for compound A, B and C. PLEASE PROVIDE CLEAR DRAWINGS AND EXPLANATIONSarrow_forwardGive the structural chemistry of active methylene, classify it asradical/intermediate/stable organic compound, reagent of its production and role in alkylationarrow_forwardA hydrocarbon (X), with the molecular formula: C8H14 is reduced in presence of sodium and liquid ammonia to give the only product (Y) with the molecular formula: C8H16. Compounds X and Y both resulting 2,5-dimethylhexane when treated with hydrogen and platinum catalyst (H2/Pt). As a result of the oxidative cleavage of compound Y (by using KMnO4 / H2SO4), a single carboxylic acid derivative with C4H8O2 molecular formula is formed. Again, as a result of the reaction of Y with perbenzoic acid, the chiral compound C8H14O is observed, but the reaction of compound Y with bromine gives the achiral C8H14Br2 as the product.arrow_forward
- Stearolic acid, C18H32O2, yields stearic acid on catalytic hydrogenation and undergoes oxidative cleavage with ozone to yield nonanoic acid and nonanedioic acid. What is the structure of stearolic acid?arrow_forwardExplain the Mechanism - Addition of Dichlorocarbene to an Alkene ?arrow_forwardCompound F may be synthesised by the method attached: When 2-chloropropane treated with NaOH and 1-chloropropane treated with NaOH separately produce two different functional groups. Provide both reactions and explain the two different functional groups produced.arrow_forward
- Propose a synthesis of the anti-inflammatory drug Ibuprofen from benzene. Show all reagents and all intermediate structures. Assume that ortho and para isomers can be separatedarrow_forwardUnsymmetrical alkene (1-butene) may be converted to Glycol by which of the following reactions options: Hydroboration in presence of hydrogen peroxide and NaOH Hydration in presence of acidic aqueous medium Oxidation with permangante Oxidation with Oxygen in presence of silver catalyst at 250 C and high pressure Ozonolysis in presence of Zinc and acidic mediumarrow_forwardOne of the products of petroleum refinery is naphtha where, benzene could beobtained via catalytic reforming of naphtha. The obtained benzene can potentiallyto react with Lewis acid to form new carbon-carbon bond. Propose the startingmaterial and stepwise mechanism to produce new chemical structure which consista formula molecule of C11H16.arrow_forward
- A nitrile compound is prepared from cyclopentene. The following is an analysis of the disconnection and its synthesis. Write the structure of A and B !arrow_forwardTwo isomers, A and B, of molecular formula C5H8 undergo catalytic hydrogenation with hydrogen gas and palladium on carbon to form the same C5H10 product. On ozonolysis followed by treatment with hydrogen peroxide (H2O2), isomer A gave a product of molecular formula C5H8O4 that has two carboxylic acid groups in it whereas isomer B gave a product of molecular formula C5H8O3 that contains a carboxylic acid group and a ketone group. Which of the following isomeric pairs best match this data?arrow_forwardTwo isomers, A and B, of molecular formula C5H8 undergo catalytic hydrogenation with hydrogen gas and palladium on carbon to form the same C5H10 product. On ozonolysis followed by treatment with hydrogen peroxide (H2O2), isomer A gave a product of molecular formula C5H8O4 that has two carboxylic acid groups in it whereas isomer B gave a product of molecular formula C5H8O3 that contains a carboxylic acid group and a ketone group. What is the isometric pair of A and B that corresponds?arrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
