
Concept explainers
8-112 Consider an initial 0.040 M hypobromous acid (HOBr) solution at a certain temperature.
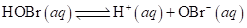
At equilibrium after partial dissociation, its pH is found to be 5.05. What is the acid ionization constant, Ka, for hypobromous acid at this temperature?
Interpretation:
The acid dissociation constant of hypobromous acid is to be calculated.
Concept Introduction:
Weak acids do not dissociate completely. Let HA be a weak acid. The dissociation of the weak acid can be represented by the chemical equation,
The equation for acid dissociation constant can be written from this chemical equation.
Here,
Answer to Problem 8.112P
The acid dissociation constant of hypobromous acid is
Explanation of Solution
Hypobromous acid is a weak acid. Hence, it do not dissociate completely. The dissociation of the given weak acid can be represented by the chemical equation,
The equation for acid dissociation constant can be written from this chemical equation.
The concentrations of each of the ions at equilibrium can be obtained from the ICE table. Where ICE represents the Initial, Change and Equilibrium concentrations of the weak acid.
The hydrogen ion concentration can be obtained from the given pH. The pH is defined as the negative logarithm of the hydrogen ion concentration.
The pH of the weak acid solution at equilibrium is 5.05. Thus, we can calculate the concentration of the hydrogen ion.
We calculated the “x” which is the concentration of hydrogen ion. The concentration of the anion is also “x”. Thus,
Now, we need to calculate the concentration of
The concentrations of the anion, hydrogen ion and hypobromous acid are used in the equation used for acid dissociation constant.
Thus, the acid dissociation constant of hypobromous acid is
Weak acids do not dissociate completely. Each weak acid has a specific dissociation constant. Here, ICE table is made from the given chemical equation. Thus, the acid dissociation constant of hypobromous acid is
Want to see more full solutions like this?
Chapter 8 Solutions
Bundle: Introduction To General, Organic And Biochemistry, 11th + Owlv2, 1 Term (6 Months) Printed Access Card
- 8-44 What is the molarity of a solution made by dissolving 3.4 g of Ba(OH)2 in enough water to make 450 mL of solution? Assume that Ba(OH)2 ionizes completely in water to Ba2+ and OH- ions. What is the pH of the solution?arrow_forward8-110 A concentrated hydrochloric acid solution contains 36.0% HCI (density 1.18 = g/mL). How many liters are required to produce 10.0 L of a solution that has a pH of 2.05?arrow_forward8-62 What is the pH of a buffer solution made by dissolving 0.10 mol of formic acid, HCOOH, and 0.10 mol of sodium formate, HCOONa, in 1 L of water?arrow_forward
- 8-60 How is the buffer capacity affected by the ratio of the conjugate base to the conjugate acid?arrow_forward8-58 What is the connection between buffer action and Le Chatelier's principle?arrow_forward8-87 The pKavalue of barbituric acid is 5.0. If the H3O+ and barbiturate ion concentrations are each 0.0030 M, what is the concentration of the undissociated barbituric acid?arrow_forward
- 8-16 For each of the following, tell whether the acid is strong or weak. (a) Acetic acid (b) HCI (c) H3PO4 (d) H2SO4 (e) HCN (f) H2CO3arrow_forward8-107 Following are pH ranges for several human biological materials. From the pH at the midpoint of each range, calculate the corresponding [H3O+]. Which materials are acidic, which are basic, and which are neutral? (a) Milk, pH 6.6-7.6 (b) Gastric contents, pH 1.0-3.0 (c) Spinal fluid, pH 7.3-7.5 (d) Saliva, pH 6.5-7.5 (e) Urine, pH 4.8-8.4 (f) Blood plasma, pH 7.35-7.45 (g) Feces, pH 4.6-8.4 (h) Bile, pH 6.8-7.0arrow_forward8-111 The volume of an adult's stomach ranges from 50 mL when empty to 1 L when full. On a certain day, its volume is 600. mL and its contents have a pH of 2.00. (a) Determine the number of moles of present. (Chapter 4) (b) Assuming that all the H+ is due to HCl(aq), how many grams of sodium hydrogen carbonate, NaHCO3, will completely neutralize the stomach acid? (Chapter 4)arrow_forward
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
