
Concept explainers
(a)
Interpretation:
The structure of chiral ether
Concept introduction:
A carbon atom that has four non-equivalent atoms or groups attached to it is known as chiral carbon atom. Chiral carbon centers are also called as asymmetric or stereogenic centers.
The ethers contain
Answer to Problem 8.30AP
The structure of chiral ether
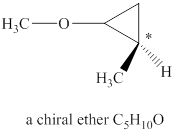
Explanation of Solution
The given molecular formula of ether is

Figure 1
The structure of chiral ether
(b)
Interpretation:
The structure of chiral alcohol with molecular formula
Concept introduction:
A carbon atom that has four non-equivalent atoms or groups attached to it is known as chiral carbon atom. Chiral carbon centers are also called as asymmetric or stereogenic centers.
The alcohols contain hydroxyl
Answer to Problem 8.30AP
The structure of chiral alcohol with molecular formula
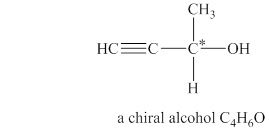
Explanation of Solution
The given molecular formula of alcohol is

Figure 2
The structure of chiral alcohol with molecular formula
(c)
Interpretation:
The structure of vicinal glycol
Concept introduction:
The alcohols contain hydroxyl
Answer to Problem 8.30AP
The structure of vicinal glycol
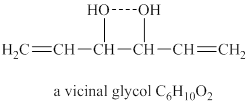
Explanation of Solution
The given molecular formula of vicinal glycol is

Figure 3
The structure of vicinal glycol
(d)
Interpretation:
The structure of diol
Concept introduction:
The alcohols contain hydroxyl
Answer to Problem 8.30AP
The structures of diol
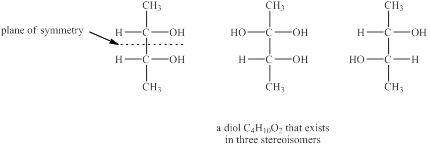
Explanation of Solution
The given molecular formula of diol is

Figure 4
The structures of diol
(e)
Interpretation:
The structure of diol
Concept introduction:
The alcohols contain hydroxyl
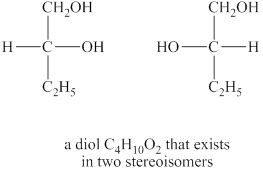
Answer to Problem 8.30AP
The structures of diol
Explanation of Solution
The given molecular formula of diol is
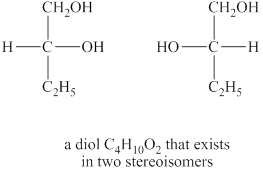
Figure 5
The structures of diol
(f)
Interpretation:
The structures of six
Concept introduction:
The ethers contain
Cyclic ethers with three-membered ring are commonly known as epoxide in which two carbons and one oxygen atom are arranged in cyclic form.
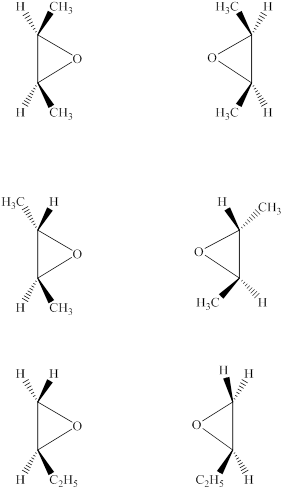
Answer to Problem 8.30AP
The structures of six epoxides (counting stereoisomers) with the molecular formula
Explanation of Solution
The given molecular formula of the epoxide is

Figure 6
The structures of six epoxides (counting stereoisomers) with the molecular formula
Want to see more full solutions like this?
Chapter 8 Solutions
ORGANIC CHEMISTRY (LL)+ SAPLING ACC >BI
- Compound A (C6H12O2) reacts with water, acid, and heatto yield compound B (C5H10O2) and compound C (CH4O).Compound B is acidic. Deduce possible structures of compounds A, B, and Carrow_forwardthe compound B C23H46,a sex attractant of the common housefly,gave two products Ch3(cH2)12COOH and CH3(CH2)7COOH. Based on this information, suggest the likely structures of Barrow_forwardCompound A (C11H23Br) is a secondary alkyl halide. On being heated with a solution of sodium ethoxide in ethanol, compound A yielded a mixture of two alkenes B and C, each having molecular formula C11H22. Catalytic hydrogenation of the major isomer B or the minor isomer C gave only 3,5-diethylheptane. Draw structures for compounds A, B, and C consistent with these observations.arrow_forward
- Determine the DOU for the following molecules and suggest a structure for each. C5H7Br2ONarrow_forwardAn unknown hydrocarbon A with the formula C6H12 reacts with 1 molar equivalent of H2 over a palladium catalyst. Hydrocarbon A also reacts with OsO4 to give diol B. When oxidized with KMnO4 in acidic solution, A gives two fragments. One fragment is propanoic acid, CH3CH2CO2H, and the other fragment is ketone C. What are the structures of A, B, and C? Write all reactions, and show your reasoning.arrow_forwardDraw the structural formula for at least one bromoalkene with the molecular formula C5H9Br that shows: Q.) Neither E,Z isomerism nor chiralityarrow_forward
- What is the degree of unsaturation for compoundC11H7ClO?arrow_forwardWhat is the degree of unsaturation and structure for C8H5NO2 ?arrow_forwardA difficult problem in the synthesis of PGF2α is the introduction of the OH group at C15 in the desired conguration.a. Label this stereogenic center as R or S.b. A well known synthesis of PGF2α involves reaction of A with Zn(BH4)2, a metal hydride reagent similar in reactivity to NaBH4, to form two isomeric products, B and C. Draw their structures and indicate their stereochemical relationship.c. Suggest a reagent to convert A to the single stereoisomer X.arrow_forward
- An unknown hydrocarbon A with the formula C6H12 reacts with 1 molar equivalent of H2 over a palladium catalyst to give hydrocarbon B. Hydrocarbon A also reacts with OsO4 to give the glycol C. When oxidized with KMnO4 in acidic solution, A gives two fragments. One fragment is propanoic acid, CH3CH2COOH, and the other fragment is ketone D (R2C=O). What are the structures of A, B, C and D? Write all reactions.arrow_forwardAspirin, or 2-acetoxybenzoic acid, (C9H8O4) is often synthesised from salicylic acid.(i) Sketch and discuss any changes in the number of possible structural conformations ofaspirin relative to those of salicylic acid. (ii) Re-draw the structure predicted to be the lowest energy conformation of aspirin,indicating any expected stabilising and destabilising interactions. Justify your choice.arrow_forwarda) When (Z)-3-methylhex-3-ene undergoes hydroboration–oxidation, two isomeric products are formed. Give their structures, and label each asymmetric carbon atom as (R) or (S). What is the relationship between these isomers?arrow_forward
