Given information:
It is given thatcycle is a regenerative cycle in a steam power plant includes one feedwater heater, turbine, condenser and a boiler. Steam enters the turbine at 650 psia and 900∘F and exhaust at 1 psia to condenser and 50 psia to feedwater heater. Steam for the feedwater heater is at 50 psia and in condensing raises the temperature of the feedwater to within 11∘F . Turbine and pump efficiencies are both given as 0.78 .
Figure to describe the process in simplified form is given as below
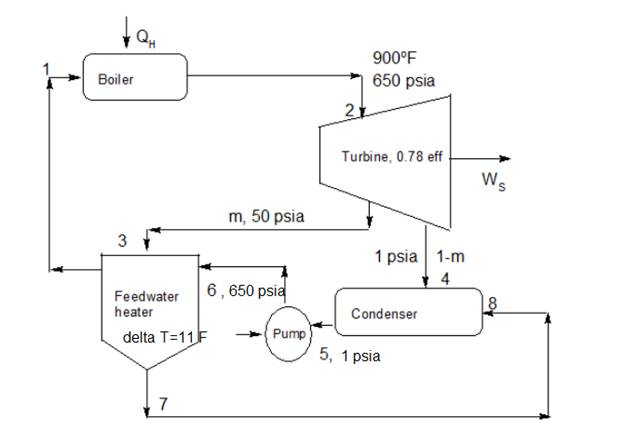
From figure point 1 and point 6 has same operating conditions.
Considering idealistic turbine for which entropy is constant at inlet and outlet
at point 2 and at 650 psia and 900∘F, the properties are written from superheated steam table in fps unit as,
H2=1461.2 Btulbm and S2=1.6671 Btulbm Rankine
Now for idealistic turbine, entropy is constant at inlet and outlet. Hence
S4'=S3'=S2=1.6671 Btulbm Rankine
Exhaust coming to condenser is at pressure 1 psia and entropy S4'=1.6671 Btulbm Rankine and turbine has thermal efficiency of 0.78 . Hence, we need to calculate the actual entropy of the steam coming to condenser inlet at point 4.
The steam coming to condenser is wet and saturated. Hence for saturated steam at 1 psia, properties are written from the steam table for the saturated steam at 1 psia as,
At 0.94924 psia
Hliq=67.999 Btulbm and Hvap=1105.1 BtulbmSliq=0.1295 Btulbm Rankine and Svap=1.9825 Btulbm Rankine
At 1.00789 psia
Hliq=69.995 Btulbm and Hvap=1105.9 BtulbmSliq=0.1331 Btulbm Rankine and Svap=1.9775 Btulbm Rankine
From linear interpolation, if M is the function of a single independent variable X then the value of M at X is intermediate between two given values, M1 at X1 and M2 at X2
M=( X 2−X X 2− X 1)M1+(X− X 1 X 2− X 1)M2Hliq=(1.00789 psia−1 psia1.00789 psia-0.94924 psia)×67.999 Btulbm+(1 psia−0.94924 psia1.00789 psia-0.94924 psia)×69.995 BtulbmHliq=69.73 Btulbm
Similarly
M=( X 2−X X 2− X 1)M1+(X− X 1 X 2− X 1)M2HVap=(1.00789 psia−1 psia1.00789 psia-0.94924 psia)×1105.1 Btulbm+(1 psia−0.94924 psia1.00789 psia-0.94924 psia)×1105.9 BtulbmHvap=1105.79 Btulbm
And
M=( X 2−X X 2− X 1)M1+(X− X 1 X 2− X 1)M2SVap=(1.00789 psia−1 psia1.00789 psia-0.94924 psia)×1.9825 Btulbm Rankine+(1 psia−0.94924 psia1.00789 psia-0.94924 psia)×1.9775 Btulbm RankineSvap=1.9781 Btulbm Rankine
M=( X 2−X X 2− X 1)M1+(X− X 1 X 2− X 1)M2Sliq=(1.00789 psia−1 psia1.00789 psia-0.94924 psia)×0.1295 Btulbm Rankine+(1 psia−0.94924 psia1.00789 psia-0.94924 psia)×0.1331 Btulbm RankineSliq=0.1326 Btulbm Rankine
And
We also know that
S4'=Sliq+x4'(Svap−Sliq)⇒x4'=S4'−SliqSvap−Sliq⇒x4'=1.6671 Btu lb m Rankine−0.1326 Btu lb m Rankine1.9781 Btu lb m Rankine−0.1326 Btu lb m Rankine⇒x4'=0.831
Therefore,
H4'=Hliq+x4'(Hvap−Hliq)H4'=69.73 Btulbm+0.831×(1105.79 Btu lb m−69.73 Btu lb m)H4'=931.195 Btulbm
Hence the actual enthalpy of the steam exhaust from the turbine to the condenser is calculated by the thermodynamics efficiency of the work produced from the turbine which is
η=WactualWideal⇒η=H4−H2H4'−H2⇒0.78=H4−1461.2 Btu lb m931.195 Btu lb m−1461.2 Btu lb m⇒H4=1047.796 Btulbm
Hence
H4=Hliq+x4(Hvap−Hliq)⇒x4=H4−HliqHvap−Hliq⇒x4=1047.796 Btu lb m−69.73 Btu lb m1105.79 Btu lb m−69.73 Btu lb m⇒x4=0.944
and
S4=Sliq+x4(Svap−Sliq)S4=0.1326 Btulbm Rankine+0.944×(1.9781 Btu lb m Rankine−0.1326 Btu lb m Rankine)S4=1.8748 Btulbm Rankine
Exhaust coming to feedwater heater is at pressure 50 psia and entropy S3'=1.6671 Btulbm Rankine and turbine has thermal efficiency of 0.78 . Hence, we need to calculate the actual entropy of the steam coming to feedwater heater inlet at point 3.
The steam coming to feedwater heater is superheated. Hence for superheated steam at 50 psia, properties are written from the steam table for the superheated steam at 50 psia as,
50 psia and S3'=1.6671 Btulbm Rankine lies in between 50 psia and S3'=1.6586 Btulbm Rankine with enthalpy 1174.1 Btulbm and 50 psia and S3'=1.6720 Btulbm Rankine with enthalpy 1184.1 Btulbm
From linear interpolation, if M is the function of a single independent variable X then the value of M at X is intermediate between two given values, M1 at X1 and M2 at X2
M=( X 2−X X 2− X 1)M1+(X− X 1 X 2− X 1)M2H3'=(1.6720 Btu lbm Rankine−1.6671 Btu lbm Rankine1.6720 Btu lbm Rankine-1.6586 Btu lbm Rankine)×1174.1 Btulbm+ (1.6671 Btu lbm Rankine−1.6586 Btu lbm Rankine1.6720 Btu lbm Rankine-1.6586 Btu lbm Rankine)×1184.1 BtulbmH3'=1180.44 Btulbm
Hence the actual enthalpy of the steam exhaust from the turbine to the feedwater heater is calculated by the thermodynamics efficiency of the work produced from the turbine which is
η=WactualWideal⇒η=H3−H2H3'−H2⇒0.78=H3−1461.2 Btu lb m1180.44 Btu lb m−1461.2 Btu lb m⇒H3=1242.21 Btulbm
And entropy at H3=1242.21 Btulbm and 50 psia from superheated steam table in fps unit after the interpolation is
S3=1.7431 Btulbm Rankine
Now,
The idealistic condenser has constant enthalpy and hence the enthalpy of saturated liquid at inlet point 4 of condenser is same as enthalpy at the outlet of condenser at point 5.
H5=Hliq=69.73 Btulbm
And at 1 psia, the volume of saturated liquid at outlet of condenser point 5 is written from the saturated steam table in fps units after the interpolation as
V5=0.0161 ft3lbm
Hence work required for circulation of the steam from the condenser to the feedwater heater is calculated fromusing the pump efficiency is
η=WidealWactualWactual=Wpump=Widealη⇒Wpump=V5( P 6− P 5)η⇒Wpump=0.0161 ft 3 lb m(650 lbf in2 −1 lbf in2 )× 12 2 in 21 ft 20.78⇒Wpump=1920.11 lbf.ftlbm×1 Btu778.17 lbf.f=2.47 Btulbm
And the work-done by pump is also calculated as
⇒Wpump=H6−H5=2.47 Btulbm⇒H6=2.47 Btulbm+69.73 Btulbm⇒H6=72.197 Btulbm
Now it is given that steam for the feedwater heater is at 50 psia and in condensing raises the temperature of the feedwater to within 11∘F .
Hence at point 7 the steam is saturated liquid from feedwater heater going towards the condenser. Hence for 50 psia and saturated steam, for saturated steam table, properties are written as,
Hliq=H7=250.21 Btulbm and Sliq=S7=0.4112 Btulbm Rankine T7=281.01∘F
Hence the temperature at the point 1 according to the given condition and in condensing raises the temperature of the feedwater to within 11∘F is
T1=281.01∘F-11∘FT1=270.01∘F
And at T1=270.01∘F, for saturated liquid from feedwater heater going towards the boiler, the properties are written from saturated steam table after interpolation as,
Hliq=238.96 Btulbm and Sliq=0.3960 Btulbm Rankine Psat=41.87 psia, Vliq=0.1717 ft3lbm
The volume expansivity for boiler is calculated as
β=1V(∂V∂T)=10.1717 ft 3 lb m×(0.01738 ft3 lbm −0.017193 ft3 lbm 292 ∘F- 272 ∘F)⇒β=5.445×10−5 1Rankine
Hence the enthalpy and entropy across the boiler is
ΔH=V(1−βT)ΔP⇒H1−Hliq=Vliq(1−βT1)(P1−Psat)H1−238.96 Btulbm=0.1717 ft3lbm×(1−(5.445× 10 −5 1 Rankine×( 270.01∘ F+459.67) Rankine)) ×(650 Psia−41.87 psia)×122 in21 ft2×1 Btu778.17 lbf.f H1=257.51 Btulbm
And
ΔS=βVΔP⇒S1−Sliq=βVliq(P1−Psat)S1−0.3960 Btulbm Rankine =0.1717 ft3lbm×5.445×10−5 1Rankine×(650 Psia−41.87 psia)×122 in21 ft2×1 Btu778.17 lbf.f S1=0.397 Btulbm Rankine
Let mass of steam exhaust from the turbine going towards feedwater heater is m hence mass of steam exhaust from turbine going towards condenser is (1−m)
Basis 1 lbm of steam exhaust of turbine
Applying energy balance across the feedwater heater. Hence fraction of the steam entering the turbine is extracted for the feedwater heater is
mH3+1 lbmH6=(1−m)H7+mH1mH3+1 lbmH6 =mH7+1 lbm H1m(H3−H7)=1 lbm (H1−H6)⇒m=H1−H6H3−H7m=257.51 Btu lb m−72.197 Btu lb m1242.21 Btu lb m−250.21 Btu lb mm=0.1868 lbm
And
The thermal efficiency of the cycle is calculated as:
η=|Wnet|QH
Here Wnet is the total work produced by the turbine and pump. QH is the difference in enthalpies of the turbine and boiler.
Wturbine-1st=η(H3'−H2)=−218.99 Btulbm×1 lbm=−218.99 BtuWturbine-2nd=(1−m)(H4−H3)=(1 lbm−0.1868 lbm)×(1047.796 Btu lb m−1242.21 Btu lb m)Wturbine-2nd=−158.098 BtuWpump=2.47 Btulbm×1 lbm=2.47 Btu⇒η=|W turbine-1st+W turbine-2nd+W pump|( H 2− H 1)⇒η=|( −218.99 Btu)+( −158.098 Btu)+2.47 Btu|(1461.2 Btu lbm −257.51 Btu lbm )⇒η=0.311



 Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education
Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
 Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning
Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The
Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The




