
Concept explainers
a)
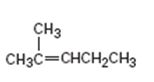
Interpretation:
The product obtained by the catalytic hydrogenation of 2-methyl-2-pentene is to be given.
Concept introduction:
To give:
The product obtained by the catalytic hydrogenation of 2-methyl-2-pentene.
b)
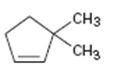
Interpretation:
The product obtained by the catalytic hydrogenation of 3,3-dimethylcyclopentene is to be given.
Concept introduction:
Alkenes react with H2 in the presence of metal catalysts such as palladium or platinum to yield the corresponding alkanes as the products. The addition occurs with syn stereochemistry. Normally only a single product is produced as the catalyst will interact only from the least hindered and most accessible face of the alkene.
To give:
The product obtained by the catalytic hydrogenation of 3,3-dimethylcyclopentene.
c)
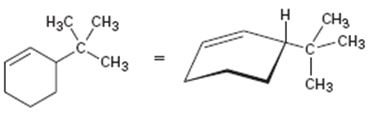
Interpretation:
The product obtained by the catalytic hydrogenation of 3-tert-butylcyclohexene is to be given.
Concept introduction:
Alkenes react with H2 in the presence of metal catalysts such as palladium or platinum to yield the corresponding alkanes as the products. The addition occurs with syn stereochemistry. Normally only a single product is produced as the catalyst will interact only from the least hindered and most accessible face of the alkene.
To give:
The product obtained by the catalytic hydrogenation of 3-tert-butylcyclohexene.
Trending nowThis is a popular solution!

Chapter 8 Solutions
Organic Chemistry
- The aryl diene undergoes sequential Heck reactions to give a product with the molecular formula C15H18. Propose a structural formula for this product.arrow_forwardAcid-catalyzed hydrolysis of the following epoxide gives a trans diol. Of the two possible trans diols, only one is formed. How do you account for this stereoselectivity?arrow_forwardPredict the product and give the stereochemistry resulting from reaction of each of the followingnucleophiles with (R)-2-bromooctanearrow_forward
- What compound is the expected product upon Markovnikov hydrohalogenation with HBr of the alkene shown below?arrow_forwardHow would you prepare the following alkyl halides, from alkenes? Write the equation for each reaction indicating substrates, reagents, and conditions.arrow_forwardHow many monohalogenation products can be obtained from the following compounds?arrow_forward

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole
Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole


