
Elements of Chemical Reaction Engineering (5th Edition) (Prentice Hall International Series in the Physical and Chemical Engineering Sciences)
5th Edition
ISBN: 9780133888096
Author: Fogler
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 9, Problem 9.10P
It has been observed that substrate inhibition occurs in the following enzymatic reaction:
E + S → P + E
- (a) Show that the rate law for substrate inhibition is consistent with the plot in Figure P9-10B of −rs (mmol/L · min) versus the substrate concentration S (mmol/L).
- (b) If this reaction is carried out in a CSTR that has a volume of 1000 dm3, to which the volumetric flow rate is 3.2 dm3/min, determine the three possible steady states, noting, if possible, which are stable. The entrance concentration of the substrate is 50 mmol/dm3. What is the highest conversion?
- (c) What would be the effluent substrate concentration if the total enzyme concentration is reduced by 33%?
- (d) List ways you can work this problem incorrectly.
- (e) How could you make this problem more difficult?
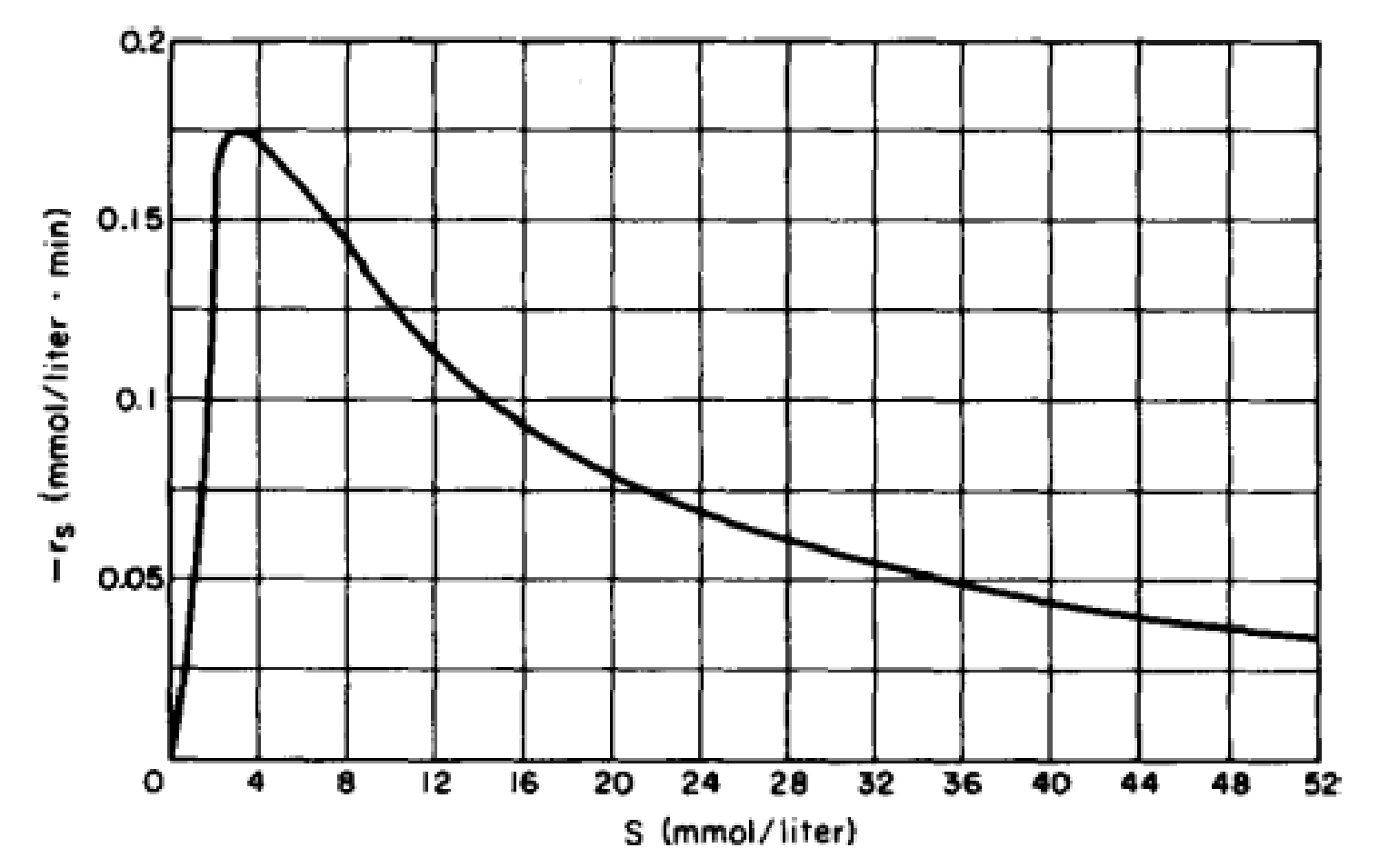
Figure P9-10B Michaelis–Menten plot for substrate inhibition.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Chapter 9 Solutions
Elements of Chemical Reaction Engineering (5th Edition) (Prentice Hall International Series in the Physical and Chemical Engineering Sciences)
Ch. 9 - (Flame retardants) Hydrogen radicals are important...Ch. 9 - The pyrolysis of acetaldehyde is believed to take...Ch. 9 - For each of the reactions in parts (a), (b), and...Ch. 9 - (Tribology) Why you change your motor oil? One of...Ch. 9 - Epidemiology. Consider the application of the PSSH...Ch. 9 - Derive the rate laws for the following enzymatic...Ch. 9 - Beef catalase has been used to accelerate the...Ch. 9 - It has been observed that substrate inhibition...Ch. 9 - The following data on bakers yeast in a particular...Ch. 9 - Prob. 9.12P
Additional Engineering Textbook Solutions
Find more solutions based on key concepts
Write and compile short programs to test the following issues: a.Determine whether your compiler will allow the...
Problem Solving with C++ (9th Edition)
A cylindrical rod with a radius of 0.3 in. and a length of 15 in. is subjected to a tensile load. If the rod is...
Materials for Civil and Construction Engineers (4th Edition)
The rigid pipe is supported by a pin at A and an A-36 steel guy wire BD. If the wire has a diameter of 0.25 in....
Mechanics of Materials (10th Edition)
Write a procedure is_little_endian that will return 1 when compiled and run on a little-endian machine, and wil...
Computer Systems: A Programmer's Perspective (3rd Edition)
An object is a(n) _____. a. blueprint b. attribute c. variable d. instance
Starting Out with Java: Early Objects (6th Edition)
Broadly speaking, what are the two main objectives of electrical systems?
Electrical Engineering: Principles & Applications (7th Edition)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemical-engineering and related others by exploring similar questions and additional content below.Recommended textbooks for you
 Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education
Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
 Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning
Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The
Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The

Introduction to Chemical Engineering Thermodynami...
Chemical Engineering
ISBN:9781259696527
Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind...
Chemical Engineering
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY

Elements of Chemical Reaction Engineering (5th Ed...
Chemical Engineering
ISBN:9780133887518
Author:H. Scott Fogler
Publisher:Prentice Hall


Industrial Plastics: Theory and Applications
Chemical Engineering
ISBN:9781285061238
Author:Lokensgard, Erik
Publisher:Delmar Cengage Learning

Unit Operations of Chemical Engineering
Chemical Engineering
ISBN:9780072848236
Author:Warren McCabe, Julian C. Smith, Peter Harriott
Publisher:McGraw-Hill Companies, The
Browning Reaction in Foods; Author: St. Teresa's College;https://www.youtube.com/watch?v=gpTUG2UlTyA;License: Standard YouTube License, CC-BY
Enzymatic Reactions: Types of Reactions & Enzymes – Biochemistry | Lecturio; Author: Lecturio Medical;https://www.youtube.com/watch?v=CI6zVmlQipU;License: Standard Youtube License