
Nitroglycerin, one of the most commonly used explosives, has the structure
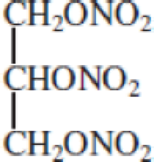
The decomposition reaction is
The explosive action is the result of the heat released and the large increase in gaseous volume. (a) Calculate the ΔH° for the decomposition of one mole of nitroglycerin using both standard enthalpy of formation values and bond enthalpies. Assume that the two O atoms in the NO2 groups are attached to N with one single bond and one double bond. (b) Calculate the combined volume of the gases at STP. (c) Assuming an initial explosion temperature of 3000 K, estimate the pressure exerted by the gases using the result from (b). (The standard enthalpy of formation of nitroglycerin is −371.1 kJ/mol.)
(a)
Interpretation:
Concept Introduction:
Change in enthalpy in a reaction and enthalpy of formation are related by the formula,
Where,
Answer to Problem 9.142QP
Explanation of Solution
The decomposition reaction is,
Reduce the equation to show the decomposition of one mole of nitroglycerin.
Dividing by 4,
Calculation of
Calculation of
| Bonds broken in reactants | Number of bonds broken | Bond enthalpy |
Enthalpy change |
| Bonds formed in product | Number of bonds formed | Bond enthalpy |
Enthalpy change |
Using the above data
(b)
Interpretation:
Combined volume of gases in the given reaction has to be calculated at STP.
Concept Introduction:
STP refers to standard temperature and pressure conditions which are
Answer to Problem 9.142QP
Combined volume of gases in the given reaction at STP is calculated as
Explanation of Solution
The decomposition reaction is,
Reduce the equation to show the decomposition of one mole of nitroglycerin.
Dividing by 4,
One mole of nitroglycerin forms
At STP, one mole of ideal gas occupies a volume of
Therefore total volume of gases in the given reaction at STP is calculated as,
(c)
Interpretation:
The pressure exerted by the gases at temperature of
Concept Introduction:
Pressure and temperature are related by ideal gas equation is,
Where,
Answer to Problem 9.142QP
The pressure exerted by the gases at temperature of
Explanation of Solution
Given data:
As we know,
Rewrite the above equation for P,
Substitute the known values.
Want to see more full solutions like this?
Chapter 9 Solutions
CHEMISTRY
- Use the appropriate tables to calculate H for (a) the reaction between copper(II) oxide and carbon monoxide to give copper metal and carbon dioxide. (b) the decomposition of one mole of methyl alcohol (CH3OH) to methane and oxygen gases.arrow_forwardThe Haber process is the synthesis of ammonia from its constituent elements: N2 (g) + 3 H2 (g) → 2 NH3 (g) ΔG ̊ > 0 at 500 ̊C Removing NH3 from the reaction vessel causes _______________.arrow_forwardCalculate the kinetic energy (in Joules) of 2.000 mole of hydrogen gas at 450.0K.arrow_forward
- 14 g of nitrogen gas were reacted with exces hydrogen. Calculate the volume of ammonia produced under standard conditions. (101 kPa, 298 K)R= 8.31 JK molarrow_forwardFrom the sequential hydrolysis of ammonia borane in 10 mL aqueous solution at 25 ºC and 1 atm with 2 mM Rh catalysis, 730.4 mL H2 was obtained in 8 hours. What is the TON of the catalyst used in the reaction?arrow_forwardGiven the following reaction of Ca(s) in HCl(aq): Ca(s) + 2 HCl(aq) --> CaCl2(aq) + H2(g) If 32.7g of calcium solid are placed in this reaction and at the end of the experiment, hydrogen gas is produced at 25.000C and with a pressure of 790. mmHg. Calculate the volume of hydrogen gas produced.arrow_forward
- Explain the Jahn-Teller effect and provide an example. (Inorganic Chemistry)arrow_forwardSua is producing margarine from natural oils such as coconut oil by hydrogenation according to the following equation C57H104O6 + 3H2 ---->C57H110O6 at 200°c and 7atm. If an industrial hydrogenator with a volume of 500 litre is charged with 24 kilograms of oil and the reaction goes to completion, how many kilograms of margarine will be produced under these conditions?arrow_forwardIn the reaction of sodium phosphate with aluminum nitrate, 65 ml of 0.125 mol/L sodium phosphate is reacted with 0.255 mol/L aluminum nitrate. What is the minimum volume of aluminum nitrate needed to remove all phosphate ions from the solution? 1. 31.9 ml 2. 63.8 ml 3. 16 ml 4. 95.7 ml 5. 10.6 mlarrow_forward
- Calcium carbide, CaC2, is manufactured by reducing lime with carbon at high temperature. (The carbide is used in turn to make acetylene, an industrially important organic chemical.) Is the reaction endothermic or exothermic?arrow_forwardGiven that a sample of air is made up of nitrogen, oxygen, and argon in the mole fractions 0.78 N2, 0.21 O2, and 0.010 Ar, what is the density of air at standard temperature and pressure?arrow_forward62 Ammonium dinitramide (ADN), NH4N(NO2)2, was considered as a possible replacement for aluminium chloride as the oxidizer in the solid fuel booster rockets used to launch the space shuttle. When detonated by a spark, AND rapidly decomposes to produce a gaseous mixture of N2,O2, and H2O. (This is not a combustion reaction. The ADN is the only reactant.) The reaction releases a lot of heat, so the gases are initially formed at high temperature and pressure. The thrust of the rocket results mainly from the expansion of this gas mixture. Suppose a 2.3-kg sample of ADN is denoted and decomposes completely to give N2,O2, and H2O. If the resulting gas mixture expands until it reaches a temperature of 100°C and a pressure of 1.00 atm, what volume will it occupy? Is your answer consistent with the proposed use of ADN as a rocket fuel?arrow_forward
 Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER
Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning





