
Concept explainers
(a)
Interpretation:
The
Concept introduction:
The compounds which have the same molecular formula but have different arrangements of atoms are known as isomers. The phenomenon is called isomerism. The isomers are generally classified as structural isomers and stereoisomers. Stereoisomers are further divided into two categories diastereomers and enantiomers.
Answer to Problem 9.44AP
The isomers of
Explanation of Solution
The structures of
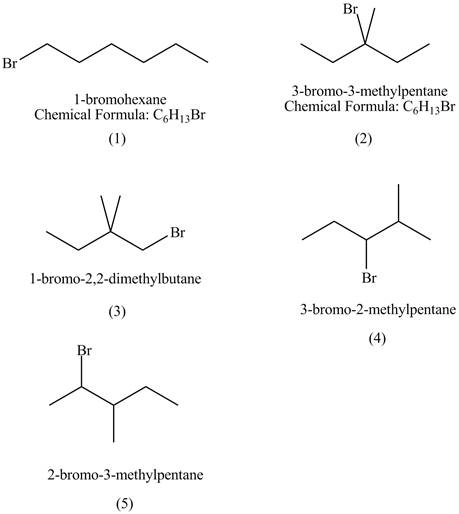
Figure 1
The compounds that have chiral centers can exist as enantiomers. The compound
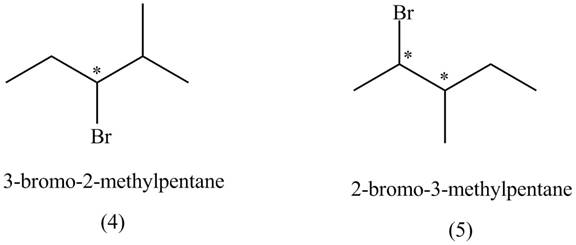
Figure 2
Therefore,
The compounds
(b)
Interpretation:
The alkyl halides among
Concept introduction:
The compounds which have the same molecular formula but have different arrangements of atoms are known as isomers. The phenomenon is called isomerism. The isomers are generally classified as structural isomers and stereoisomers. Stereoisomers are further divided into two categories diastereomers and enantiomers.
Answer to Problem 9.44AP
The isomer of
Explanation of Solution
The structures of
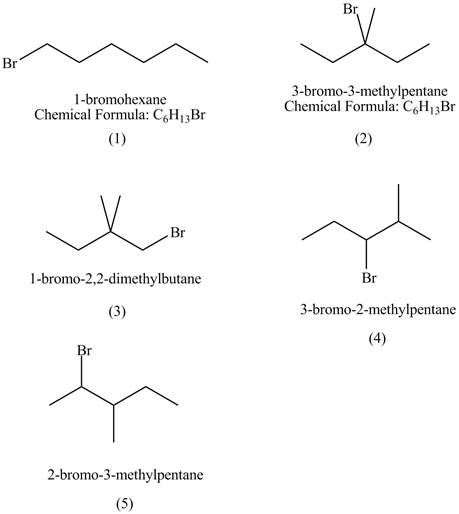
Figure 1
The compounds that have two chiral centers can exist as enantiomers. The compound
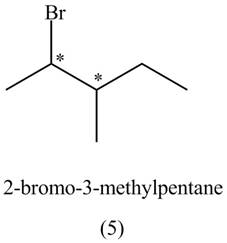
Figure 3
Therefore,
The compound
(c)
Interpretation:
The alkyl halides among
Concept introduction:
The replacement or substitution of one functional group with another different functional group in any chemical reaction is termed as a substitution reaction. In a nucleophilic substitution reaction, nucleophile takes the position of leaving the group by attacking on the electron-deficient carbon atom.
Answer to Problem 9.44AP
The fastest
Explanation of Solution
The structures of
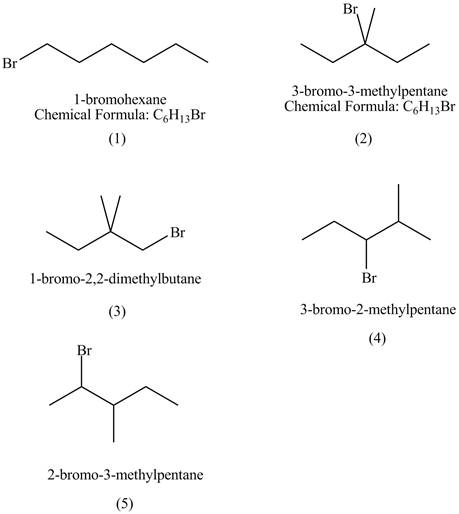
Figure 1
The rate of
The compound
The compound
(d)
Interpretation:
The alkyl halides among
Concept introduction:
The replacement or substitution of one functional group with another different functional group in any
Answer to Problem 9.44AP
The isomer of
Explanation of Solution
The structures of

Figure 1
The compound
The compound,
(e)
Interpretation:
The alkyl halides among
Concept introduction:
The elimination reaction of alkyl halide involves removal of the halogen atom and hydrogen atom from the adjacent carbon atoms, which leads to the formation of the alkene. A bulky base increases the chance of elimination reaction of substitution reaction.
Answer to Problem 9.44AP
The compound
Explanation of Solution
The structures of
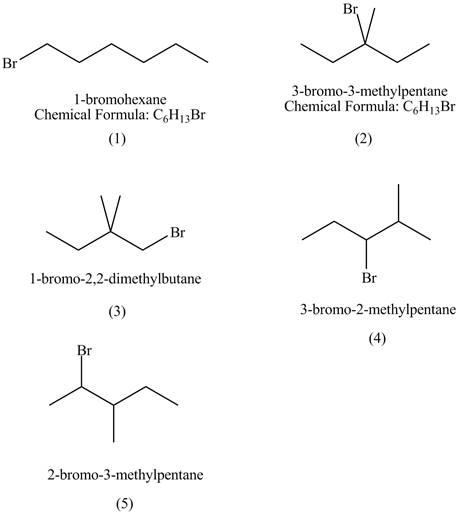
Figure 1
The compound
The
(f)
Interpretation:
The alkyl halides among
Concept introduction:
The replacement or substitution of one functional group with another different functional group in any chemical reaction is termed as a substitution reaction. In a nucleophilic substitution reaction, nucleophile takes the position of leaving the group by attacking on the electron-deficient carbon atom.
Answer to Problem 9.44AP
The alkyl halide among
Explanation of Solution
The structures of
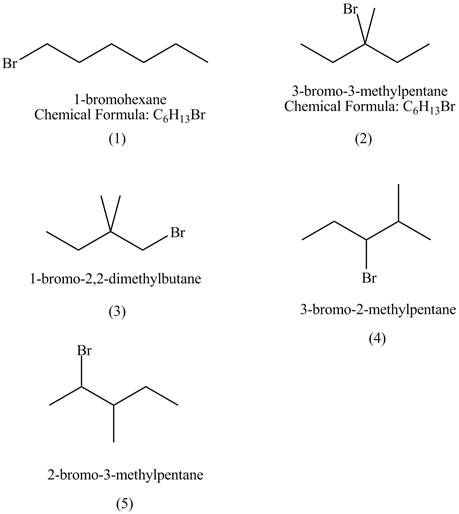
Figure 1
The rate of
The steric hindrance in case of
The isomer of
(g)
Interpretation:
The alkyl halides among
Concept introduction:
The replacement or substitution of one functional group with another different functional group in any chemical reaction is termed as a substitution reaction. In a nucleophilic substitution reaction, nucleophile takes the position of leaving the group by attacking on the electron-deficient carbon atom.
Answer to Problem 9.44AP
The alkyl halides among
Explanation of Solution
The structures of

Figure 1
The stability of tertiary carbocation is more than the stability of secondary carbocation. Therefore, in case of
The isomer of
(h)
Interpretation:
The alkyl halide among
Concept introduction:
The replacement or substitution of one functional group with another different functional group in any chemical reaction is termed as a substitution reaction. In a nucleophilic substitution reaction, nucleophile takes the position of leaving the group by attacking on the electron-deficient carbon atom.
Answer to Problem 9.44AP
The alkyl halide among
Explanation of Solution
The structures of

Figure 1
The rate of
Therefore,
The isomer of
Want to see more full solutions like this?
Chapter 9 Solutions
Organic Chemistry 6e & Study Guide
- Drawing on what you know about the stereochemistry of alkene addition reactions, a. write the mechanism for the reaction of 2-butyne with one equivalent of Br2. b. predict the configuration of the product of the reaction.arrow_forward3. Which of the following compounds would you expect to have the greatest density: hexane,1-bromohexane, 1-fluorohexane, or 1-chlorohexane? Explain your answer.arrow_forwardRank the following groups in order of decreasing priority. −Cl, −CH3, −SH, −OHarrow_forward
- Sight along the C2-Cl bond of 2-methylpropane (isobutane). a. Draw a Newman projection of the most stable conformation. b. Draw a Newman projection of the least stable conformation. c. Make a graph of energy versus angle of rotation around the C2-Cl bond. d. Assign relative values to the maxima and minima in your graph, given that an H↔H eclipsing interaction costs 0 kJ/mol and an H↔CH3 eclipsing interaction costs 6.0 kJ/mol.arrow_forwardConsider 2-methylbutane (isopentane). Looking along the C2-C3 bond: a) Draw a Newmann projection of the most stable conformation. b) Draw a Newmann projection of the least stable conformation. c) Indicate the gauche form. d) State the eclipsed Newmann projection. An eclipsed CH3-CH3 interaction has an energy value of 11 kJ / mol and a gauche CH3-CH3 interaction has an energy value of 3.8 kJ / mol, the data are given if you need them.arrow_forwardHow many alkenes or cycloalkenes with the general formula C9H16 can you treat with HCl to obtain 1-chloro-1-ethyl-3,4-dimethylcyclopentane as a major product?arrow_forward
- Construct a model of 1,2-dibromoethane, BrCH2CH2Br. Examine the possible conformations of this molecule. Which of the following interactions will be the greatest? a. hydrogen-hydrogen interactions b. hydrogen-bromine interactions c. bromine-bromine interactionsarrow_forward1. name the following compund ( see attachment) 2. Solve for the value of x in the following combustion reaction of a particular alkane in the presence of heat, CH3(CH2)xCH3 + yO2 → zCO2 + 14 H2O A. 4 B. 3 C. 15 D. 11 E. 5arrow_forwardI have a question about calculating the elements of unsaturation in C8H19N. When I solve the equation, I get a negative number. What am I doing wrong?arrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

