
Concept explainers
(a)
Interpretation:
If the given statement is true or false should be determined.
Concept Introduction:
Isotopes which are having balance numbers of protons and neutrons are stable but the serious imbalance can lead to nuclear reaction. Radioactive isotopes emit alpha, beta, positron, gamma particles due to nuclear reactions.
- Alpha emission is an emission of helium nucleus (2 Protons and 2 neutrons) from the elements to stabilize the nucleus. This emission results into decrease by two in
atomic number and increase by four in mass number. - Beta emission is an emission of beta particle as electron from nucleus of molecule. This emission results into increase in atomic number and no change in mass number.
- Positron emission is an emission in electron with positive charge is emitted by nucleus of molecule. This emission results into decrease by one number in atomic number and no change in mass number.
- Gamma radiation is only access energy from nucleus is emitted to stabilize nucleus of molecule. This emission results into no change in mass number and atomic number.
These emitted particles are useful in diagnosis and treatment of diseases as a nuclear medicine. They act as invaluable tool in fieled of science. These nuclear chemistry is utilized in medicla imaging like PET (Positron emission tomography), X-rays, MRI (Magnetic resonance Imaging), CT scan etc.
Answer to Problem 9.50P
Of the radioisotopes listed in Table-9.5, the majority decay by gamma radiation.Thus, the statement is false.
Explanation of Solution
Table-9.5 contains the lists of some radioactive isotopes which are used in medical imaging. As per the data provided in table, there are maximum numbers of radioactive isotopes that emits gamma radiation.
(b)
Interpretation:
If the given statement is true or false should be determined.
Concept Introduction:
Isotopes which are having balance numbers of protons and neutrons are stable, but the serious imbalance can lead to nuclear reaction. Radioactive isotopes emit alpha, beta, positron, gamma particles due to nuclear reactions.
- Alpha emission is an emission of helium nucleus (2 Protons and 2 neutrons) from the elements to stabilize the nucleus. This emission results into decrease by two in atomic number and increase by four in mass number.
- Beta emission is an emission of beta particle as electron from nucleus of molecule. This emission results into increase in atomic number and no change in mass number.
- Positron emission is an emission in electron with positive charge is emitted by nucleus of molecule. This emission results into decrease by one number in atomic number and no change in mass number.
- Gamma radiation is only access energy from nucleus is emitted to stabilize nucleus of molecule. This emission results into no change in mass number and atomic number.
These emitted particles are useful in diagnosis and treatment of diseases as a nuclear medicine. They act as invaluable tool in fieled of science. These nuclear chemistry is utilized in medicla imaging like PET (Positron emission tomography), X-rays, MRI (Magnetic resonance Imaging), CT scan etc.
Answer to Problem 9.50P
Isotopes that decay by alpha emission are rarely if ever used in nuclear imaging because alpha emitters are rare.Thus, the statement is true.
Explanation of Solution
Table-9.5 with the list of radioactive isotopes used in medical imaging contains no radioactive isotope that emits alpha particles. There are very rare isotopes useful in medical like radium-223 for treatment of cancer.
(c)
Interpretation:
If the given statement is true or false should be determined.
Concept Introduction:
Isotopes which are having balance numbers of protons and neutrons are stable, but the serious imbalance can lead to nuclear reaction. Radioactive isotopes emit alpha, beta, positron, gamma particles due to nuclear reactions.
- Alpha emission is an emission of helium nucleus (2 Protons and 2 neutrons) from the elements to stabilize the nucleus. This emission results into decrease by two in atomic number and increase by four in mass number.
- Beta emission is an emission of beta particle as electron from nucleus of molecule. This emission results into increase in atomic number and no change in mass number.
- Positron emission is an emission in electron with positive charge is emitted by nucleus of molecule. This emission results into decrease by one number in atomic number and no change in mass number.
- Gamma radiation is only access energy from nucleus is emitted to stabilize nucleus of molecule. This emission results into no change in mass number and atomic number.
These emitted particles are useful in diagnosis and treatment of diseases as a nuclear medicine. They act as invaluable tool in fieled of science. These nuclear chemistry is utilized in medicla imaging like PET (Positron emission tomography), X-rays, MRI (Magnetic resonance Imaging), CT scan etc.
Answer to Problem 9.50P
Gamma emitters are so widely used in medical imaging because gamma radiation is penetrating and, therefore, can easily be measured by radiation detectors outside the body.Thus, the statement is true.
Explanation of Solution
Gamma emitter are widely used because of gamma particles had no mass and charge and they can easily penetrate in body.
(d)
Interpretation:
If the given statement is true or false should be determined.
Concept Introduction:
Isotopes which are having balance numbers of protons and neutrons are stable, but the serious imbalance can lead to nuclear reaction. Radioactive isotopes emit alpha, beta, positron, gamma particles due to nuclear reactions.
- Alpha emission is an emission of helium nucleus (2 Protons and 2 neutrons) from the elements to stabilize the nucleus. This emission results into decrease by two in atomic number and increase by four in mass number.
- Beta emission is an emission of beta particle as electron from nucleus of molecule. This emission results into increase in atomic number and no change in mass number.
- Positron emission is an emission in electron with positive charge is emitted by nucleus of molecule. This emission results into decrease by one number in atomic number and no change in mass number.
- Gamma radiation is only access energy from nucleus is emitted to stabilize nucleus of molecule. This emission results into no change in mass number and atomic number.
These emitted particles are useful in diagnosis and treatment of diseases as a nuclear medicine. They act as invaluable tool in fieled of science. These nuclear chemistry is utilized in medicla imaging like PET (Positron emission tomography), X-rays, MRI (Magnetic resonance Imaging), CT scan etc.
Answer to Problem 9.50P
When selenium-75 deays by electron capture and gamma emission, the new nucleus formed is arsenic-75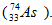 ).Thus, the statement is false.
).Thus, the statement is false.
Explanation of Solution
When selenium-75 decays by electron capture, a new nuclues formed should contain 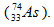
(e)
Interpretation:
If the given statement is true or false should be determined.
Concept Introduction:
Isotopes which are having balance numbers of protons and neutrons are stable, but the serious imbalance can lead to nuclear reaction. Radioactive isotopes emit alpha, beta, positron, gamma particles due to nuclear reactions.
- Alpha emission is an emission of helium nucleus (2 Protons and 2 neutrons) from the elements to stabilize the nucleus. This emission results into decrease by two in atomic number and increase by four in mass number.
- Beta emission is an emission of beta particle as electron from nucleus of molecule. This emission results into increase in atomic number and no change in mass number.
- Positron emission is an emission in electron with positive charge is emitted by nucleus of molecule. This emission results into decrease by one number in atomic number and no change in mass number.
- Gamma radiation is only access energy from nucleus is emitted to stabilize nucleus of molecule. This emission results into no change in mass number and atomic number.
These emitted particles are useful in diagnosis and treatment of diseases as a nuclear medicine. They act as invaluable tool in fieled of science. These nuclear chemistry is utilized in medicla imaging like PET (Positron emission tomography), X-rays, MRI (Magnetic resonance Imaging), CT scan etc.
Answer to Problem 9.50P
When iodine-131 decays by beta and gamma emission, the new nucleus formed is xenon-131 (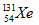 ).Thus, the statement is true.
).Thus, the statement is true.
Explanation of Solution
During the decay of iodine-131 by beta and gamma emission, the new nucleus formed contains one number more in atomic mass. This new nucleus is Xenon-131 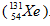
(f)
Interpretation:
If the given statement is true or false should be determined.
Concept Introduction:
Isotopes which are having balance numbers of protons and neutrons are stable, but the serious imbalance can lead to nuclear reaction. Radioactive isotopes emit alpha, beta, positron, gamma particles due to nuclear reactions.
- Alpha emission is an emission of helium nucleus (2 Protons and 2 neutrons) from the elements to stabilize the nucleus. This emission results into decrease by two in atomic number and increase by four in mass number.
- Beta emission is an emission of beta particle as electron from nucleus of molecule. This emission results into increase in atomic number and no change in mass number.
- Positron emission is an emission in electron with positive charge is emitted by nucleus of molecule. This emission results into decrease by one number in atomic number and no change in mass number.
- Gamma radiation is only access energy from nucleus is emitted to stabilize nucleus of molecule. This emission results into no change in mass number and atomic number.
These emitted particles are useful in diagnosis and treatment of diseases as a nuclear medicine. They act as invaluable tool in fieled of science. These nuclear chemistry is utilized in medicla imaging like PET (Positron emission tomography), X-rays, MRI (Magnetic resonance Imaging), CT scan etc.
Answer to Problem 9.50P
In positron emission tomography (a PET scan), the detectors counts the numbers of gamma particle emitted by a tagged material and the location within the body where the tagged materail accumulates. Thus, the statement is false.
Explanation of Solution
The radiation contains particles with energy which are emitted by radioactive isotopes. This energy is useful to trace the particle within the body during its medical use. The Positron Tomography Scan contains Positrons which has very short live and they collide with electron and forms gamma rays. PET scanner capture the energy of the particles and it does not count the numbers of positrons.
(g)
Interpretation:
If the given statement is true or false should be determined.
Concept Introduction:
Isotopes which are having balance numbers of protons and neutrons are stable, but the serious imbalance can lead to nuclear reaction. Radioactive isotopes emit alpha, beta, positron, gamma particles due to nuclear reactions.
- Alpha emission is an emission of helium nucleus (2 Protons and 2 neutrons) from the elements to stabilize the nucleus. This emission results into decrease by two in atomic number and increase by four in mass number.
- Beta emission is an emission of beta particle as electron from nucleus of molecule. This emission results into increase in atomic number and no change in mass number.
- Positron emission is an emission in electron with positive charge is emitted by nucleus of molecule. This emission results into decrease by one number in atomic number and no change in mass number.
- Gamma radiation is only access energy from nucleus is emitted to stabilize nucleus of molecule. This emission results into no change in mass number and atomic number.
These emitted particles are useful in diagnosis and treatment of diseases as a nuclear medicine. They act as invaluable tool in fieled of science. These nuclear chemistry is utilized in medicla imaging like PET (Positron emission tomography), X-rays, MRI (Magnetic resonance Imaging), CT scan etc.
Answer to Problem 9.50P
The use of 18-fluorodeoxyglucose (FDG) in PET scans of the brain depends on the fact that FDG behaves in the body as does glucose.Thus, the statement is true.
Explanation of Solution
The use of 18-fluorodeoxyglucose is as glucose in the body because the six carbons of glucose are replaced by fluorine-18 and it can easily cross the Blood-brain barrier.
(h)
Interpretation:
If the given statement is true or false should be determined.
Concept Introduction:
Isotopes which are having balance numbers of protons and neutrons are stable, but the serious imbalance can lead to nuclear reaction. Radioactive isotopes emit alpha, beta, positron, gamma particles due to nuclear reactions.
- Alpha emission is an emission of helium nucleus (2 Protons and 2 neutrons) from the elements to stabilize the nucleus. This emission results into decrease by two in atomic number and increase by four in mass number.
- Beta emission is an emission of beta particle as electron from nucleus of molecule. This emission results into increase in atomic number and no change in mass number.
- Positron emission is an emission in electron with positive charge is emitted by nucleus of molecule. This emission results into decrease by one number in atomic number and no change in mass number.
- Gamma radiation is only access energy from nucleus is emitted to stabilize nucleus of molecule. This emission results into no change in mass number and atomic number.
These emitted particles are useful in diagnosis and treatment of diseases as a nuclear medicine. They act as invaluable tool in fieled of science. These nuclear chemistry is utilized in medicla imaging like PET (Positron emission tomography), X-rays, MRI (Magnetic resonance Imaging), CT scan etc.
Answer to Problem 9.50P
A goal of raditaion therapy is to destroy pathological cells and tissues without at the same time damaging normal cells and tissues.Thus, the statement is true.
Explanation of Solution
The radiation therapy has a goal to just destroy pathological cells and tissues but inevitably it also damages healthy and normal cells and tissue that surrounds damaged tissues.
(i)
Interpretation:
If the given statement is true or false should be determined.
Concept Introduction:
Isotopes which are having balance numbers of protons and neutrons are stable, but the serious imbalance can lead to nuclear reaction. Radioactive isotopes emit alpha, beta, positron, gamma particles due to nuclear reactions.
- Alpha emission is an emission of helium nucleus (2 Protons and 2 neutrons) from the elements to stabilize the nucleus. This emission results into decrease by two in atomic number and increase by four in mass number.
- Beta emission is an emission of beta particle as electron from nucleus of molecule. This emission results into increase in atomic number and no change in mass number.
- Positron emission is an emission in electron with positive charge is emitted by nucleus of molecule. This emission results into decrease by one number in atomic number and no change in mass number.
- Gamma radiation is only access energy from nucleus is emitted to stabilize nucleus of molecule. This emission results into no change in mass number and atomic number.
These emitted particles are useful in diagnosis and treatment of diseases as a nuclear medicine. They act as invaluable tool in fieled of science. These nuclear chemistry is utilized in medicla imaging like PET (Positron emission tomography), X-rays, MRI (Magnetic resonance Imaging), CT scan etc.
Answer to Problem 9.50P
In external beam radition, radiation from an external source is directed at a tissue from outside the body.Thus, the statement is false.
Explanation of Solution
In external beam radiation, a high energy beam is used to target the tumor cells from outside the body. It destroys the cells which are available inside the body and not the surface of body.
(j)
Interpretation:
If the given statement is true or false should be determined.
Concept Introduction:
Isotopes which are having balance numbers of protons and neutrons are stable, but the serious imbalance can lead to nuclear reaction. Radioactive isotopes emit alpha, beta, positron, gamma particles due to nuclear reactions.
- Alpha emission is an emission of helium nucleus (2 Protons and 2 neutrons) from the elements to stabilize the nucleus. This emission results into decrease by two in atomic number and increase by four in mass number.
- Beta emission is an emission of beta particle as electron from nucleus of molecule. This emission results into increase in atomic number and no change in mass number.
- Positron emission is an emission in electron with positive charge is emitted by nucleus of molecule. This emission results into decrease by one number in atomic number and no change in mass number.
- Gamma radiation is only access energy from nucleus is emitted to stabilize nucleus of molecule. This emission results into no change in mass number and atomic number.
These emitted particles are useful in diagnosis and treatment of diseases as a nuclear medicine. They act as invaluable tool in fieled of science. These nuclear chemistry is utilized in medicla imaging like PET (Positron emission tomography), X-rays, MRI (Magnetic resonance Imaging), CT scan etc.
Answer to Problem 9.50P
In internal beam radiation, a radioactive material is implnated in a target tissue to destroy cells in the target tissue without doing appreciable damage to surrounding normla tissues.Thus, the statement is true.
Explanation of Solution
In internal beam radiation therapy, a radioactive material is implanted into body which is located very near to the tumor cells or tissues. This type of radiation does not make more damage to the surrounding tissues or cells.
Want to see more full solutions like this?
Chapter 9 Solutions
Introduction to General, Organic and Biochemistry
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning




