
Concept explainers
Draw the products of each reaction.
a.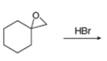 c.
c.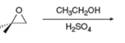
b. 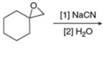 d.
d. 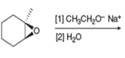
(a)
Interpretation: The product of the given reaction is to be drawn.
Concept introduction: The opening of an epoxide/ethylene oxide ring is regioselective either it takes place with a strong nucleophile
Answer to Problem 9.65P
The product of the given reaction is,
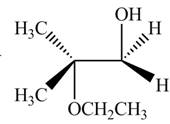
Explanation of Solution
The given reaction involves treatment of unsymmetrical epoxide with
The opening of an epoxide/ethylene oxide ring is regioselective either it takes place with a strong nucleophile
Thus, the product of the given reaction is,
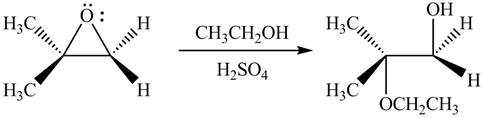
Figure 1
The product of the given reaction is drawn in Figure 1.
(b)
Interpretation: The product of the given reaction is to be drawn.
Concept introduction: The opening of an epoxide/ethylene oxide ring is regioselective either it takes place with a strong nucleophile
Answer to Problem 9.65P
The product of the given reaction is,
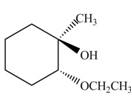
Explanation of Solution
The given reaction involves treatment of unsymmetrical epoxide with
The opening of an epoxide/ethylene oxide ring is regioselective either it takes place with a strong nucleophile
Thus, the product of the given reaction is,
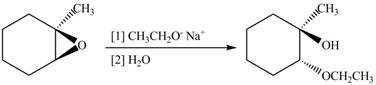
Figure 2
The product of the given reaction is drawn in Figure 2.
(c)
Interpretation: The product of the given reaction is to be drawn.
Concept introduction: The opening of an epoxide/ethylene oxide ring is regioselective either it takes place with a strong nucleophile
Answer to Problem 9.65P
The product of the given reaction is,
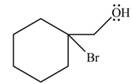
Explanation of Solution
The given reaction involves treatment of unsymmetrical epoxide with
The opening of an epoxide/ethylene oxide ring is regioselective either it takes place with a strong nucleophile
Thus, the product of the given reaction is,
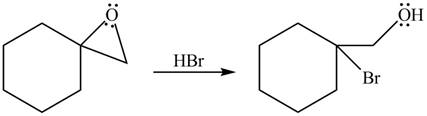
Figure 3
The product of the given reaction is drawn in Figure 3.
(d)
Interpretation: The product of the given reaction is to be drawn.
Concept introduction: The opening of an epoxide/ethylene oxide ring is regioselective either it takes place with a strong nucleophile
Answer to Problem 9.65P
The product of the given reaction is,
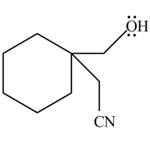
Explanation of Solution
The given reaction involves treatment of unsymmetrical epoxide with
The opening of an epoxide/ethylene oxide ring is regioselective either it takes place with a strong nucleophile
Thus, the product of the given reaction is,
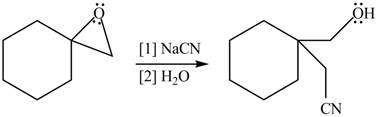
Figure 4
The product of the given reaction is drawn in Figure 4.
Want to see more full solutions like this?
Additional Science Textbook Solutions
Organic Chemistry (9th Edition)
Chemistry: A Molecular Approach (4th Edition)
Organic Chemistry - Standalone book
Chemistry: Matter and Change
Introduction to Chemistry
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
