
Organic Chemistry (9th Edition)
9th Edition
ISBN: 9780321971371
Author: Leroy G. Wade, Jan W. Simek
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 12, Problem 12.24SP
covered a synthesis of
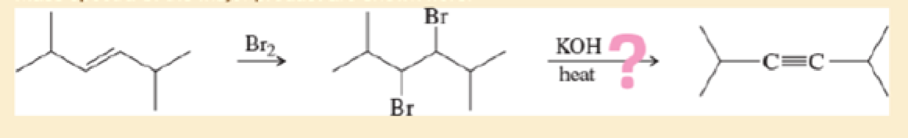
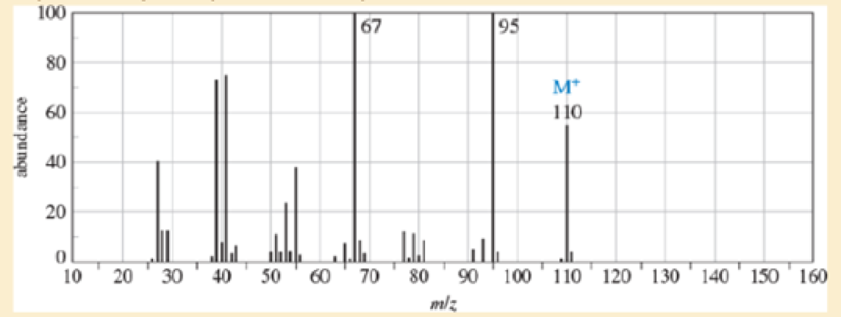
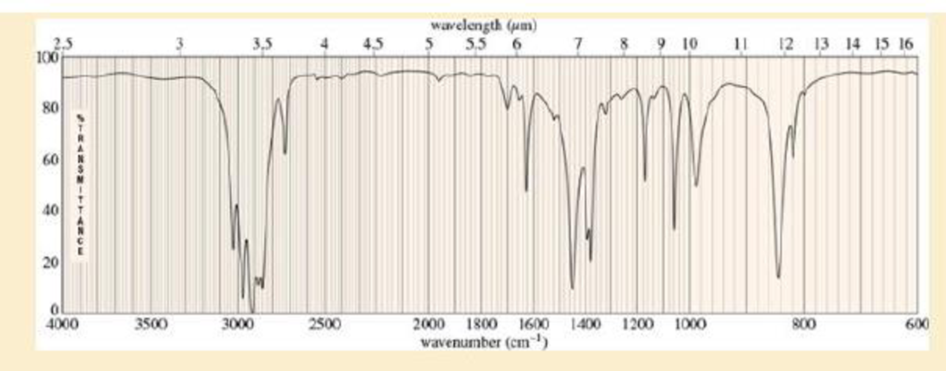
- a. Do the spectra confirm the right product? If not, what is it?
- b. Explain the important peaks in the IR spectrum.
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
Why we need step 3 before step 4?
a. Because the nitro group increases the electrophilicity at the ortho positions which is where the bromine is added.
b. Because the amine group is a strong ortho, para director which is what controls the regiochemical outcome of this bromination.
c. Step 4 is unessesary. The symmetry of compound 3 allows for the bromination to be regioselective and give compound 5.
5. There will be a mixture of products because there is no selectivity for a major product.
Explain why the following reaction is not a good way to prepare 1-phenylpentane:
benzene + 1-chloropentane + AlCl3 → 1-phenylpentane
In your scratch work, give the full, curved-arrow mechanistic explanation of what would happen, and indicate what would likely be your main product or products.
When the allylic alkyl bromide shown below is heated in ethanol solvent, the major elimination product isolated is the diene shown below.
A) Propose a mechanism to account for this overall transformation. Use normal curved arrows to show movement of electron pairs and be sure to draw structures for all important reaction intermediates. If an intermediate is a resonance hybrid, draw all important contributing resonance structures.
B) The same alkyl bromide shown above also yields several substitution products when subjected the reaction conditions described above. Draw the structure of the major substitution product that would be isolated in the box on the product side of the reaction arrow.
The second picture is for the B part of the question
Chapter 12 Solutions
Organic Chemistry (9th Edition)
Ch. 12.3 - Complete the following conversion table. (cm1)...Ch. 12.5 - Which of the bonds shown in red are expected to...Ch. 12.7C - For each hydrocarbon spectrum, determine whether...Ch. 12.9A - Spectra are given for three compounds. Each...Ch. 12.10 - The infrared spectra for three compounds are...Ch. 12.12 - Prob. 12.6PCh. 12.14B - Identify which of these four mass spectra indicate...Ch. 12.15A - Show the fragmentation that accounts for the...Ch. 12.15A - Show the fragmentations that give rise to the...Ch. 12.15B - Ethers are not easily differentiated by their...
Ch. 12.15C - Prob. 12.11PCh. 12 - Prob. 12.12SPCh. 12 - Prob. 12.13SPCh. 12 - All of the following compounds absorb infrared...Ch. 12 - Prob. 12.15SPCh. 12 - Four infrared spectra are shown, corresponding to...Ch. 12 - Predict the masses and the structures of the most...Ch. 12 - Prob. 12.18SPCh. 12 - Prob. 12.19SPCh. 12 - (A true story) While organizing the undergraduate...Ch. 12 - Prob. 12.21SPCh. 12 - Prob. 12.22SPCh. 12 - An unknown, foul-smelling hydrocarbon gives the...Ch. 12 - covered a synthesis of alkynes by a double...Ch. 12 - Three IR spectra are shown, corresponding to three...Ch. 12 - Prob. 12.26SPCh. 12 - Prob. 12.27SPCh. 12 - Prob. 12.28SPCh. 12 - The ultimate test of fluency in MS and IR is...Ch. 12 - Prob. 12.30SPCh. 12 - Consider the following four structures, followed...
Additional Science Textbook Solutions
Find more solutions based on key concepts
The active ingredient in Tylenol and a host of other over-the-counter pain relievers is acetaminophen (C8H9NO2)...
Chemistry: Atoms First
Determine [OH], [H+], and the pH of each of the following solutions. a. 1.0 M KCl b. 1.0 M KC2H3O2
Chemistry
Practice Exercise 1
Which of the following factors determines the size of an atom? a. the volume of the nucleus...
Chemistry: The Central Science (13th Edition)
Practice Problem ATTEMPT
Write the rate expressions for each of the following reactions:
(a)
(b)
(c)
Chemistry
Q1. What is the empirical formula of a compound with the molecular formula
Chemistry: A Molecular Approach
4. 38 Strontium has four naturally occurring isotopes, with mass numbers 84, 86, 87, arid 88.
a. Write the atom...
Basic Chemistry (5th Edition)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Construct an efficient three-step synthesis of 1,2-epoxycyclopentane from bromocyclopentane by dragging the appropriate formulas into the bins. Note that each bin will hold only one item, and not all of the given reagents or structures will be used.arrow_forwardOrganic chemistry student Everett has set up two separate electrophilic addition reactions with 2-butyne (shown below). In reaction (i) the alkyne is treated with 1 equivalent of HBr, which successful provides (Z)-2-bromo-2-butene. In reaction (ii) the alkyne is subjected to a two-step hydroboroation/oxidation sequence. Interestingly, Everett’s second reaction fails to provide the desired methyl ketone and instead has provided butan-2,3-diol. (a) Provide the structure of intermediate X form the first step of reaction (ii). (b) Briefly explain (use structures if necessary) why reaction (i) provided an alkene where as reaction (ii) provided an alkane when only 1 equivalent of the electrophile was used in each reaction.arrow_forwardGive a detailed mechanism including all resonance isomers for intermediates (show electron flow) for the Friedel-Crafts reaction done in the lab. NOTE: It is likely the mechanism from reactants to only 1-t-butyl-2,5-dimethoxybenzene is on the blackboard in class. I want a complete mechanism showing all steps and resonance isomers for the synthesis of the dialkylated product!arrow_forward
- Find the products for these two reactions. Make sure to include relevant hydrogens, deuteriums and stereohemistry.arrow_forwardWhen carrying out this two step procedure (bromination and subsequent elimination) on cyclopentene, the lab students were surprised to find out that an alkyne was not the final product. What would the final product most likely be?arrow_forwardStep 5b: Add curved arrows to show formation of the productarrow_forward
- Help me understand the break down of this reaction in sequence and a complete reaction scheme, thank you!arrow_forwardWrite down the mechanism of the reactions given below and find the resulting product.arrow_forwardPlease I need help with this question Based on the experiment- Synthesis of Iodosalicylamide – An Electrophilic AromaticSubstitution (a derivative of benzene, salicylamide was used and substituted an iodine atom onto the ring; the ring is the nucleophile while the atom/molecule that is being substituted onto the ring has anelectrophilic character) Please I need help with this QUESTION: Draw the structure of the final product(s) based on the ATTACHED IR analysis. Also label the signals (both the frequency and molecular motion) that help determine the final product(s). Thanks in advancearrow_forward
- A student carried out the preceding reaction, but stopped it before it was half over, whereupon he isolated the major product. He was surprised to find that the product he isolated was neither of the products obtained when the reaction was allowed to go to completion. What product did he isolate?arrow_forwardHelp ASAP Illustrate the resonance effect of the methoxy group -OCH3, on the structure of the benzene ring. Do this by writing all the possible resonance forms for methoxybenzene, including the hybrid. Based on your structures, explain how the presence of the -OCH3 group affects: (i) the reactivity of the benzene ring towards electrophilic attack; (ii) the orientation or point of attack of an incoming electrophilic reagent on the benzene ring.arrow_forwardAny help with this would be great! Explanations definitely welcome, thanks in advance for any help:) What is the major product of the reactions?arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning
Chapter 4 Alkanes and Cycloalkanes Lesson 2; Author: Linda Hanson;https://www.youtube.com/watch?v=AL_CM_Btef4;License: Standard YouTube License, CC-BY
Chapter 4 Alkanes and Cycloalkanes Lesson 1; Author: Linda Hanson;https://www.youtube.com/watch?v=PPIa6EHJMJw;License: Standard Youtube License