
a)

Interpretation:
The product formed when 5-decyne reacts with H2 in the presence of Lindlar catalyst.
Concept introduction:
To predict:
The product formed when 5-decyne reacts with H2 in the presence of Lindlar catalyst.
Answer to Problem 32AP
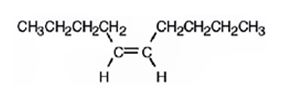
The product formed when 5-decyne reacts with H2 in the presence of Lindlar catalyst is cis-5-decene
Explanation of Solution
When 5-decyne reacts with hydrogen in the presence of Lindlar catalyst, the addition of hydrogens to both carbons in triple bond takes place resulting in the formation of cis-5-decene.
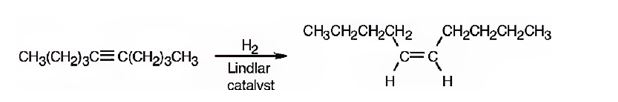
The product expected when 5-decyne reacts with H2 in the presence of Lindlar catalyst is cis-5-decene.

b)

Interpretation:
The product formed when 5-decyne reacts with Li in ammonia.
Concept introduction:
Alkynes can be reduced by treatment with hydrogen in the presence of a catalyst. The hydrogenation occurs with anti stereochemistry to give a trans-alkene as product if Li in ammonia is used.
To predict:
The product formed when 5-decyne reacts with Li in ammonia.
Answer to Problem 32AP
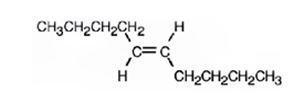
The product formed when 5-decyne reacts with Li in ammonia is trans-5-decene.
Explanation of Solution
When 5-decyne reacts with hydrogen in the presence of Li in ammonia, the addition of hydrogens to both carbons in triple bond takes place following antistereochemistry resulting in the formation of trans-5-decene.
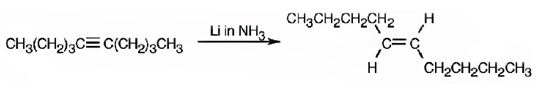
The product expected when 5-decyne reacts with Li in ammonia is trans-5-decene.

c)
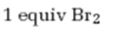
Interpretation:
The product formed when 5-decyne reacts with one equivalent of bromine.
Concept introduction:
Alkynes undergo addition reactions with anti stereochemistry when treated with bromine. When treated with one equivalent of bromine a trans-dibromoalkene results as product by the addition of bromine atoms to the carbons in triple bond from opposite faces.
To predict:
The product formed when 5-decyne reacts with one equivalent of bromine.
Answer to Problem 32AP
The product formed when 5-decyne reacts with one equivalent of bromine is trans-5,6-dibromo-5-hexene.
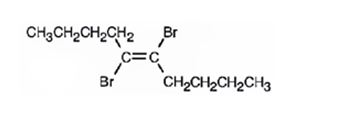
Explanation of Solution
When 5-decyne reacts with bromine, the addition of bromine to both carbons in triple bond takes place following anti stereochemistry, resulting in the formation of trans-5,6-dibromo-5-decene as the product.
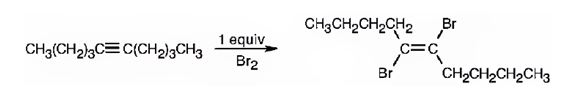
The product formed when 5-decyne reacts with one equivalent of bromine is trans-5,6-dibromo-5-decene.

d)
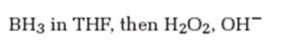
Interpretation:
The product formed when 5-decyne reacts with BH3 in THF followed by with H2O2, OH-.
Concept introduction:
Alkynes get hydrated in the hydroboration oxidation reaction. The reaction occurs with syn stereochemistry following anti Markovnikov regiochemistry to produce an enol. The enol then tautomerizes to yield a
To predict:
The product formed when 5-decyne reacts with BH3 in THF followed by with H2O2, OH-.
Answer to Problem 32AP
The product formed when 5-decyne reacts with BH3 in THF followed by with H2O2, OH- is 6-decanone.
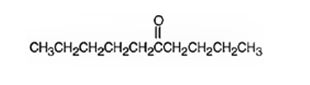
Explanation of Solution
When 5-decyne is treated with BH3 in THF followed by with H2O2, OH-, the reaction occurs with syn stereochemistry following anti Markovnikov regiochemistry to produce an enol. The enol then tautomerizes to yield a ketone as the product. The Boron and hence the OH group adds to the less highly substituted carbon in triple bond and H adds to the more highly substituted carbon atom in the triple bond to produce an enol. The enol then tautomerizes to yield the ketone as the product.
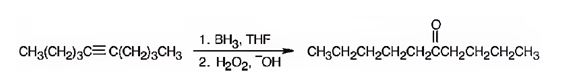
The product formed when 5-decyne reacts with BH3 in THF followed by with H2O2, OH-is 6-decanone.

e)
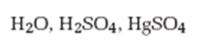
Interpretation:
The product formed when 5-decyne reacts with H2O, H2SO4 in the presence of HgSO4 is to be given.
Concept introduction:
When treated with H2O, H2SO4 in the presence of HgSO4 alkynes get hydrated to produce enols. The addition of water to unsymmetrical alkynes follows Markonikov regiochemistry while it cannot be applied in the case of symmetrical alkynes. The enols produced then undergo tautomerization to give a ketone as the product.
To predict:
The product formed when 5-decyne reacts with H2O, H2SO4 in the presence of HgSO4.
Answer to Problem 32AP
The product formed when 5-decyne reacts with H2O, H2SO4 in the presence of HgSO4 is 5-decanone.
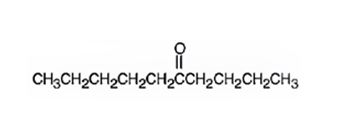
Explanation of Solution
5-Decyne is a symmetrical alkyne. Hence Markonikov regiochemistry is not applicable. When treated with dilute H2SO4 in the presence of HgSO4, the OH group adds to a carbon in triple bond and H adds to the other carbon in triple bond to yield an enol. The enol then tautomerizes to yield the ketone, 5-decanone.

The product formed when 5-decyne reacts with H2O, H2SO4 in the presence of HgSO4 is 5-decanone.

f)
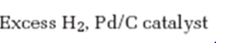
Interpretation:
The product formed when 5-decyne reacts with excess H2 in the presence of Pd/C is to be given.
Concept introduction:
When treated with excess H2, Pd/C catalyst the alkynes react with two molar equivalents of hydrogen to give the parent
To predict:
The product formed when 5-decyne reacts with excess H2 in the presence of Pd/C.
Answer to Problem 32AP
The product formed when 5-decyne reacts with excess H2 in the presence of Pd/C is n-decane.
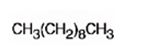
Explanation of Solution
When 5-Decyne is treated with excess H2 in the presence of Pd/C, two molar equivalents of hydrogens add to the triple bond. The corresponding alkane, n-decane is the product.
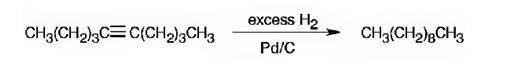
The product formed when 5-decyne reacts with excess H2 in the presence of Pd/C is n-decane.
Want to see more full solutions like this?
Chapter 9 Solutions
Organic Chemistry - Owlv2 Access (4 Term)
- -Ocimene is a pleasant-smelling hydrocarbon found in the leaves of certain herbs. It has the molecular formula C10H16 and a UV absorption maximum at 232 nm. On hydrogenation with a palladium catalyst, 2,6-dimethyloctane is obtained. Ozonolysis of -ocimene, followed by treatment with zinc and acetic acid, produces the following four fragments: (a) How many double bonds does -ocimene have? (b) Is -ocimene conjugated or nonconjugated? (c) Propose a structure for -ocimene. (d) Write the reactions, showing starting material and products.arrow_forwardCompound A (C9H12) absorbed 3 equivalents of H2 on catalytic reduction over apalladium catalyst to give B (C9H18). On ozonolysis, compound A produced 3 products, C, Dand E which was a ketone identified as cyclohexanone. On treatment with NaNH2 in NH3,followed by addition of iodomethane, compound A gave new hydrocarbon, F (C10H14). Drawstructures of A, B, C, D and F.arrow_forwardCompound A (C7H11Br) is treated with magnesium in ether to give B (C7H11MgBr) which reacts violently with D2O to give 1-methylcyclohexene with a deuterium atom on the methyl group (C). Reaction of B with acetone (Ch3COCH3) followed by hydrolysis gives D (C10H18O). Heating D with concentrated H2SO4 gives E (C10H16), which reacts with 2 equivalents of Br2 to give F (C10H16Br4). E undergoes hydrogenation with excess H2 and Pt catalyst to give 2-methylpropylcyclohexane. Determine the structures of compounds A through F, and show your reasoning throughout.arrow_forward
- 2-bromo-2-methylbutane undergoes hydrolysis reaction with water, H2O toform compound W. Compound X and compound Y are produced when 2-bromo-2-methylbutane undergoes elimination reaction with alcoholic ofsodium hydroxide, NaOH. (i) Draw the structural formula of compounds W, X and Yarrow_forwardCompound A (C7H11Br) is treated with magnesium in ether to give B (C7H11MgBr), which reacts violently with D2O to give 1-methylcyclohexene with a deuterium atom on the methyl group (C). Reaction of B with acetone (CH3COCH3) followed by hydrolysis gives D (C10H18O). Heating D with concentrated H2 SO4 gives E (C10 H16), which decolorizes two equivalents of Br2 to give F (C10H16 Br4). E undergoes hydrogenation with excess H2 and a Pt catalyst to give isobutylcyclohexane. Determine the structures of compounds A through F, and show your reasoning throughout.arrow_forwardDeduce the structure of each compound from the information given. All unknowns in this problem have molecularformula C8H12.(a) Upon catalytic hydrogenation, unknown W gives cyclooctane. Ozonolysis of W, followed by reduction with dimethylsulfide, gives octanedioic acid, HOOC¬(CH2)6¬COOH. Draw the structure of Warrow_forward
- A hydrocarbon (X), with the molecular formula: C8H14 is reduced in presence of sodium and liquid ammonia to give the only product (Y) with the molecular formula: C8H16. Compounds X and Y both resulting 2,5-dimethylhexane when treated with hydrogen and platinum catalyst (H2/Pt). As a result of the oxidative cleavage of compound Y (by using KMnO4 / H2SO4), a single carboxylic acid derivative with C4H8O2 molecular formula is formed. Again, as a result of the reaction of Y with perbenzoic acid, the chiral compound C8H14O is observed, but the reaction of compound Y with bromine gives the achiral C8H14Br2 as the product.arrow_forwardDeduce the structure of each compound from the information given. All unknowns in this problem have molecularformula C8H12.(a) Upon catalytic hydrogenation, unknown W gives cyclooctane. Ozonolysis of W, followed by reduction with dimethylsulfide, gives octanedioic acid, HOOC¬(CH2)6¬COOH. Draw the structure of W.(b) Upon catalytic hydrogenation, unknown X gives cyclooctane. Ozonolysis of X, followed by reduction with dimethylsulfide, gives two equivalents of butanedial, O“CH¬CH2CH2¬CH“O. Draw the structure of X.(c) Upon catalytic hydrogenation, unknown Y gives cyclooctane. Ozonolysis of Y, followed by reduction with dimethylsulfide, gives a three-carbon dialdehyde and a five-carbon dialdehyde. Draw the structure of Y.arrow_forwardDeduce the structure of each compound from the information given. All unknowns in this problem have molecularformula C8H12.(a) Upon catalytic hydrogenation, unknown W gives cyclooctane. Ozonolysis of W, followed by reduction with dimethylsulfide, gives octanedioic acid, HOOC¬(CH2)6¬COOH. Draw the structure of W.(b) Upon catalytic hydrogenation, unknown X gives cyclooctane. Ozonolysis of X, followed by reduction with dimethylsulfide, gives two equivalents of butanedial, O“CH¬CH2CH2¬CH“O. Draw the structure of X.(c) Upon catalytic hydrogenation, unknown Y gives cyclooctane. Ozonolysis of Y, followed by reduction with dimethylsulfide, gives a three-carbon dialdehyde and a five-carbon dialdehyde. Draw the structure of Y.*(d) Upon catalytic hydrogenation, unknown Z gives cis-bicyclo[4.2.0]octane. Ozonolysis of Z, followed by reductionwith dimethyl sulfide, gives a cyclobutane with a three-carbon aldehyde (¬CH2¬CH2¬CHO) group on C1 and aone-carbon aldehyde (¬CHO) group on C2. Draw the…arrow_forward
- When 1-butanol is treated with conc. H2SO4 and heat .A product 'A' is formed from the reaction. Treatment of 'A' with HCl/H2O gives 'B'. Treatment of 'A' with cold KMnO4/OH- gives 'C' Treatment of 'A' with hot KMnO4 /OH- gives 'D' followed by acidification of the mixture to give 'E' 1. identify the compounds represented by A,B,C,D and E.arrow_forward1. Compound A, C9H12, absorbs 3 equivalents of H2 on catalytic hydrogenation over palladium catalyst to give B, C9H18. On the treatment with acidic KMnO4, compound A gives among other things, a ketone that was identified as cyclohexanone. On reaction with NaNH2 in NH3, followed by addition of iodomethane, compound A gives a new hydrocarbon C, C10H14. What are the structures of A , B and C?arrow_forward1. write out all the isomers of the compound with molecular formula C4H10O. 2. select the normal/primary isomer and treat it with conc.H2SO4 and heat to produce A. Identify the type of reaction 3. Treatment of A with HCl/H2O gives B and with cold KMnO4/ OH- gives C . Give the name and structures of A, B and C 4. Treatment of A with Hot KMnO4/OH- gives D followed by acidification of the mixture to give E. What is D and E ?arrow_forward

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

