1 Figure 14.23 Heterogeneous Hydrogen catalysis. Mechanism for reaction of ethylene with hydrogen on a catalytic surface. Carbon H2 and C2H4 adsorb on metal surface. After H-H bond breaks, H atoms migrate along metal surface. One free H attaches to C2H4 to form C2H5 (ethyl group) intermediate. Second free H is about to attach to C2H5 intermediate to form C2Hg. Ethane, C2H6, desorbs from metal surface.
1 Figure 14.23 Heterogeneous Hydrogen catalysis. Mechanism for reaction of ethylene with hydrogen on a catalytic surface. Carbon H2 and C2H4 adsorb on metal surface. After H-H bond breaks, H atoms migrate along metal surface. One free H attaches to C2H4 to form C2H5 (ethyl group) intermediate. Second free H is about to attach to C2H5 intermediate to form C2Hg. Ethane, C2H6, desorbs from metal surface.
Organic Chemistry: A Guided Inquiry
2nd Edition
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Andrei Straumanis
Chapter15: Radical Reactions
Section: Chapter Questions
Problem 20CTQ
Related questions
Question
The first step in the heterogeneous
hydrogenation of ethylene is adsorption of the
ethylene molecule on a metal surface. One proposed explanation for the “sticking” of ethylene to a metal
surface is the interaction of the electrons in the C—C π
bond with vacant orbitals on the metal surface. (a) If this
notion is correct, would ethane be expected to adsorb to a metal surface, and, if so, how strongly would ethane bind
compared to ethylene? (b) Based on its Lewis structure,
would you expect ammonia to adsorb to a metal surface
using a similar explanation as for ethylene?
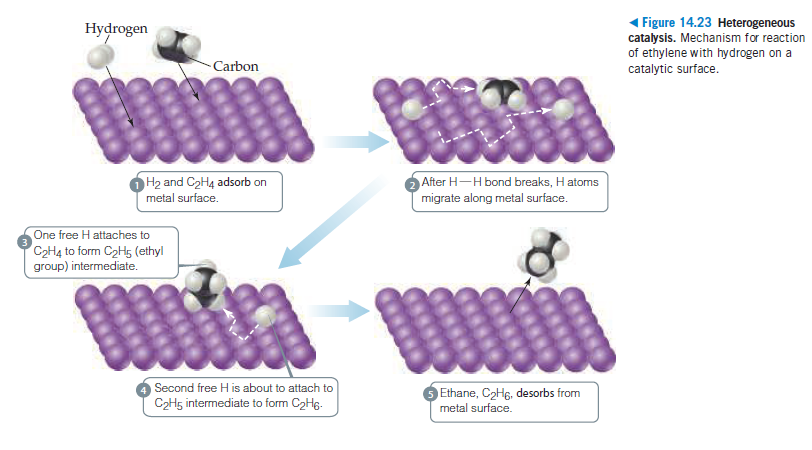
Transcribed Image Text:1 Figure 14.23 Heterogeneous
Hydrogen
catalysis. Mechanism for reaction
of ethylene with hydrogen on a
catalytic surface.
Carbon
H2 and C2H4 adsorb on
metal surface.
After H-H bond breaks, H atoms
migrate along metal surface.
One free H attaches to
C2H4 to form C2H5 (ethyl
group) intermediate.
Second free H is about to attach to
C2H5 intermediate to form C2Hg.
Ethane, C2H6, desorbs from
metal surface.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning