1. Molarity of the NaOH solution 0.238 mol/L Trial 1 Trial 2 Trial 2. Volume of H;PO4 added to flask 22.0 mL 22.0 mL 22.0 mL 3. Initial NaOH volume _0.25_ mL _0.75_ mL 0.55 mL 4. Final NaOH volume 17.60_ mL 18.20_ mL _17.90_ mL 5. NAOH volume used for titration to reach green end point mL mL mL 6. NaOH volume used for titration 7. Moles of NaOH used for titration mol mel mel 8. Moles of H;PO4 that reacted mol mel mol 9. Volume of H;PO4 added to flask L 10. Molarity of H3PO4 mol/L mol/L mol/L
1. Molarity of the NaOH solution 0.238 mol/L Trial 1 Trial 2 Trial 2. Volume of H;PO4 added to flask 22.0 mL 22.0 mL 22.0 mL 3. Initial NaOH volume _0.25_ mL _0.75_ mL 0.55 mL 4. Final NaOH volume 17.60_ mL 18.20_ mL _17.90_ mL 5. NAOH volume used for titration to reach green end point mL mL mL 6. NaOH volume used for titration 7. Moles of NaOH used for titration mol mel mel 8. Moles of H;PO4 that reacted mol mel mol 9. Volume of H;PO4 added to flask L 10. Molarity of H3PO4 mol/L mol/L mol/L
Introductory Chemistry: A Foundation
9th Edition
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Donald J. DeCoste
Chapter15: Solutions
Section: Chapter Questions
Problem 45QAP
Related questions
Question
100%
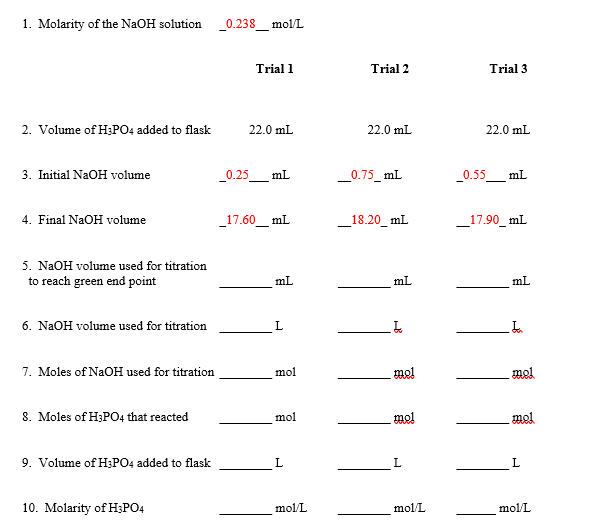
Transcribed Image Text:1. Molarity of the NaOH solution
0.238
mol/L
Trial 1
Trial 2
Trial
2. Volume of H;PO4 added to flask
22.0 mL
22.0 mL
22.0 mL
3. Initial NaOH volume
_0.25_ mL
_0.75_ mL
0.55 mL
4. Final NaOH volume
17.60_ mL
18.20_ mL
_17.90_ mL
5. NAOH volume used for titration
to reach green end point
mL
mL
mL
6. NaOH volume used for titration
7. Moles of NaOH used for titration
mol
mel
mel
8. Moles of H;PO4 that reacted
mol
mel
mol
9. Volume of H;PO4 added to flask
L
10. Molarity of H3PO4
mol/L
mol/L
mol/L
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Recommended textbooks for you

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning


Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning