A. Stoichiometric Point B. End point C. Precision D. Titer AB. Neutralization ABC. Standard Solution AC. Acidimetry AD. Alkalimetry AE. Titrant BD. Volumetric Analysis BE. Secondary Standard CD. Indicator ABD. Standardization ABE. Direct Titration ACD. Residual Titration ACE. Primary Standard E. Accuracy BC. Analyte CE. Selectivity DE. Robustness 1. A process wherein a substance is directly determined by titration to an end point with a standard solution. 2. It is said to be a sample being analyzed in a certain titrimetric procedure. 3. This is the process of measuring a basic sample by titration with a standard acid. 4. A type of analysis done by dissolving a sample ina measured quantity of standard solution known to be in excess and back titrating the excess solution with another standard solution. 5. Described as the weight of a substance which is chemically equivalent to 1 ml of standard solution. 6. It is a solution of known concentration which is prepared and standardized for titrimetric analysis. 7. It pertains to a reaction with an acid and a base resulting to the formation of salt and water. 8. It is the theoretical in which the added titrant is chemically equivalent to the analyte. 9. It is an alternative to a primary standard used for standardization of titrants. 10. A solid substance of known purity that is used in preparing standard solutions. 11. An organic compound that signals the end of the titration process. 12. It is the capacity of a titrimetric method to remain unaffected from small deviations. 13. Amethod of analysis involving the determination of a solution of known concentration required to react with a given amount of substance to be analyzed
A. Stoichiometric Point B. End point C. Precision D. Titer AB. Neutralization ABC. Standard Solution AC. Acidimetry AD. Alkalimetry AE. Titrant BD. Volumetric Analysis BE. Secondary Standard CD. Indicator ABD. Standardization ABE. Direct Titration ACD. Residual Titration ACE. Primary Standard E. Accuracy BC. Analyte CE. Selectivity DE. Robustness 1. A process wherein a substance is directly determined by titration to an end point with a standard solution. 2. It is said to be a sample being analyzed in a certain titrimetric procedure. 3. This is the process of measuring a basic sample by titration with a standard acid. 4. A type of analysis done by dissolving a sample ina measured quantity of standard solution known to be in excess and back titrating the excess solution with another standard solution. 5. Described as the weight of a substance which is chemically equivalent to 1 ml of standard solution. 6. It is a solution of known concentration which is prepared and standardized for titrimetric analysis. 7. It pertains to a reaction with an acid and a base resulting to the formation of salt and water. 8. It is the theoretical in which the added titrant is chemically equivalent to the analyte. 9. It is an alternative to a primary standard used for standardization of titrants. 10. A solid substance of known purity that is used in preparing standard solutions. 11. An organic compound that signals the end of the titration process. 12. It is the capacity of a titrimetric method to remain unaffected from small deviations. 13. Amethod of analysis involving the determination of a solution of known concentration required to react with a given amount of substance to be analyzed
Principles of Instrumental Analysis
7th Edition
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
ChapterA1: Evaluation Of Analytical Data
Section: Chapter Questions
Problem A1.22QAP
Related questions
Question
Choose the answers from the box
Please answer 4,5,6,7,8
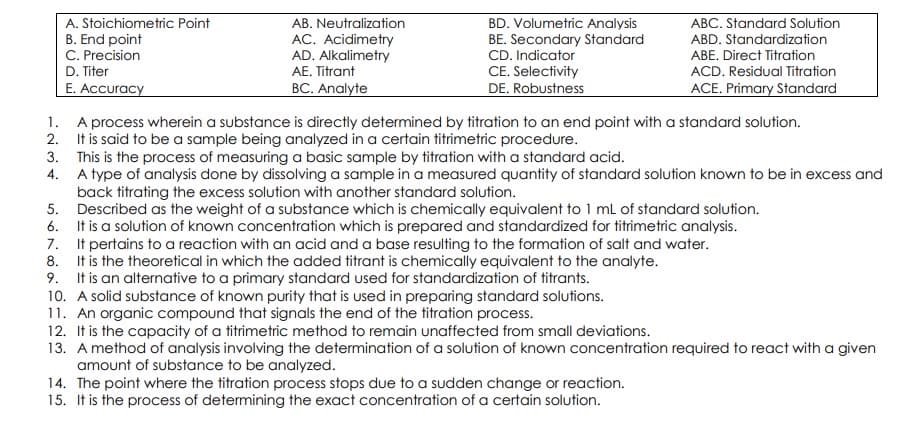
Transcribed Image Text:BD. Volumetric Analysis
BE. Secondary Standard
CD. Indicator
CE. Selectivity
DE. Robustness
A. Stoichiometric Point
AB. Neutralization
ABC. Standard Solution
B. End point
C. Precision
D. Titer
E. Accuracy
AC. Acidimetry
AD. Alkalimetry
AE. Titrant
ABD. Standardization
ABE. Direct Titration
ACD. Residual Titration
ACE. Primary Standard
BC. Analyte
1. A process wherein a substance is directly determined by titration to an end point with a standard solution.
2. It is said to be a sample being analyzed in a certain titrimetric procedure.
3. This is the process of measuring a basic sample by titration with a standard acid.
4. A type of analysis done by dissolving a sample ina measured quantity of standard solution known to be in excess and
back titrating the excess solution with another standard solution.
5. Described as the weight of a substance which is chemically equivalent to 1 ml of standard solution.
6. It is a solution of known concentration which is prepared and standardized for titrimetric analysis.
7. It pertains to a reaction with an acid and a base resulting to the formation of salt and water.
8. It is the theoretical in which the added titrant is chemically equivalent to the analyte.
9. It is an alternative to a primary standard used for standardization of titrants.
10. A solid substance of known purity that is used in preparing standard solutions.
11. An organic compound that signals the end of the titration process.
12. It is the capacity of a titrimetric method to remain unaffected from small deviations.
13. A method of analysis involving the determination of a solution of known concentration required to react with a given
amount of substance to be analyzed.
14. The point where the titration process stops due to a sudden change or reaction.
15. It is the process of determining the exact concentration of a certain solution.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning



Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

