1. NaN3 (s) → Na (s) + N2 (g) Provided above is the UNBALANCED decomposition reaction of sodium azide, NaN3 used to inflate safety airbag. Determine the amount (in gram) of NaN3 required to be used if the target volume of N, gas is 6.6 L. Given the airbag is tested in standard temperature and pressure (STP) condition.
1. NaN3 (s) → Na (s) + N2 (g) Provided above is the UNBALANCED decomposition reaction of sodium azide, NaN3 used to inflate safety airbag. Determine the amount (in gram) of NaN3 required to be used if the target volume of N, gas is 6.6 L. Given the airbag is tested in standard temperature and pressure (STP) condition.
Chapter7: Statistical Data Treatment And Evaluation
Section: Chapter Questions
Problem 7.19QAP
Related questions
Question
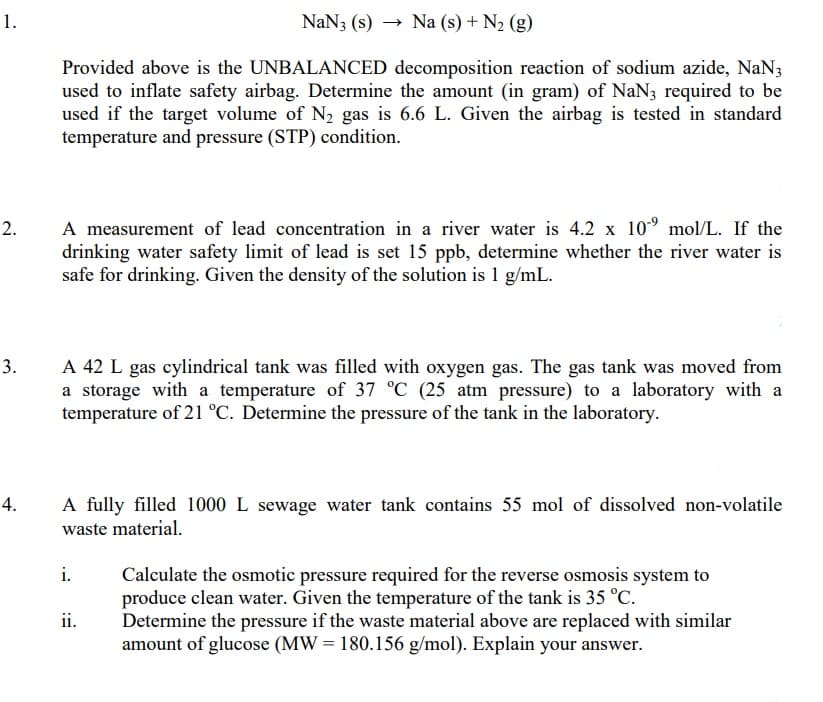
Transcribed Image Text:1.
NaN3 (s)
- Na (s) + N2 (g)
Provided above is the UNBALANCED decomposition reaction of sodium azide, NaN3
used to inflate safety airbag. Determine the amount (in gram) of NaN3 required to be
used if the target volume of N2 gas is 6.6 L. Given the airbag is tested in standard
temperature and pressure (STP) condition.
A measurement of lead concentration in a river water is 4.2 x 10° mol/L. If the
drinking water safety limit of lead is set 15 ppb, determine whether the river water is
safe for drinking. Given the density of the solution is 1 g/mL.
2.
A 42 L gas cylindrical tank was filled with oxygen gas. The gas tank was moved from
a storage with a temperature of 37 °C (25 atm pressure) to a laboratory with a
temperature of 21 °C. Determine the pressure of the tank in the laboratory.
3.
A fully filled 1000 L sewage water tank contains 55 mol of dissolved non-volatile
waste material.
i.
Calculate the osmotic pressure required for the reverse osmosis system to
produce clean water. Given the temperature of the tank is 35 °C.
Determine the pressure if the waste material above are replaced with similar
amount of glucose (MW = 180.156 g/mol). Explain your answer.
ii.
4.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning



General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning