12. When elements from Group A combine with elements from Group C, these- compounds form- unalied oldslinvs boasoo A non-conductive metal 21, 2H.0 B liquid slo olor C insert metals nt of water protPE art ahoqu D salts 13. Which element is a noble gas? gn * nitrogen (N) vanadium (V) E astatine (At) atom that is espons bonding of aloms is helium (He)
12. When elements from Group A combine with elements from Group C, these- compounds form- unalied oldslinvs boasoo A non-conductive metal 21, 2H.0 B liquid slo olor C insert metals nt of water protPE art ahoqu D salts 13. Which element is a noble gas? gn * nitrogen (N) vanadium (V) E astatine (At) atom that is espons bonding of aloms is helium (He)
Introductory Chemistry: A Foundation
9th Edition
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Donald J. DeCoste
Chapter4: Chemical Foundations: Elements, Atoms, And Ions
Section: Chapter Questions
Problem 8QAP: The symbols for most elements are based on the first few letters of the respective element’s common...
Related questions
Question
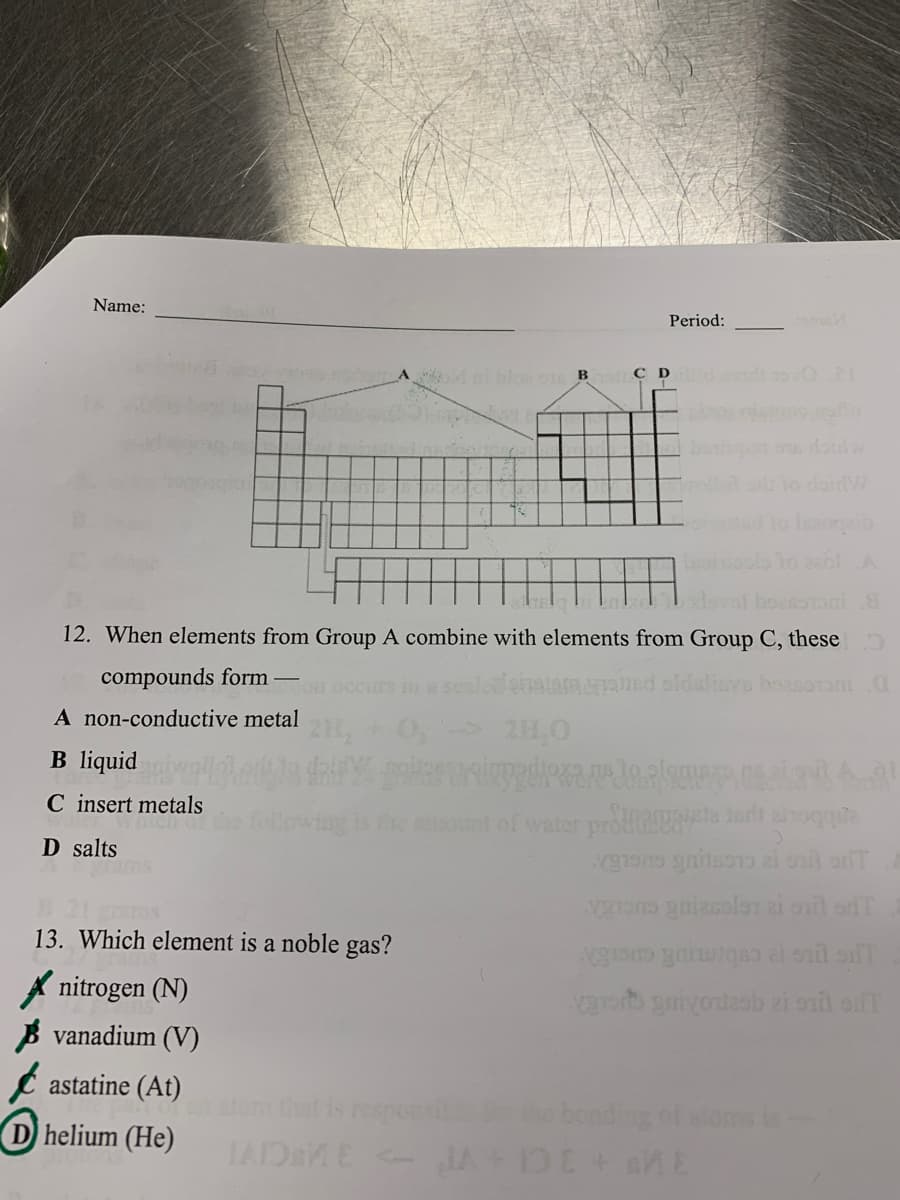
Transcribed Image Text:Name:
Period:
Mnbloo B
C D nd 1oO2I
Isoi
1o 201A
lovof boessoni
12. When elements from Group A combine with elements from Group C, these
compounds form
lied oldslinvs boasoroni.O
Fon
A non-conductive metal
2H,0
B liquid
C insert metals
hich of
ont of water proflie ari anoqque
D salts
13. Which element is a noble gas?
A nitrogen (N)
B vanadium (V)
e astatine (At)
atom that is respons
bonding of aloms is
D helium (He)
IAIDaME - JA
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Living By Chemistry: First Edition Textbook
Chemistry
ISBN:
9781559539418
Author:
Angelica Stacy
Publisher:
MAC HIGHER

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning