19. Does the name 2-butene indicate which of the stereoisomers above is being referred to? 20. To indicate the difference between alkenes that have 2 substituents across a double bond, use the prefix trans (across the double bond relative to each other) or cis (same side of the double bond t each other). Thus, the correct IUPAC names for the compounds above are cis-2-butene and trans butene. 21. Add cis or trans where needed to the IUPAC names in the alkene CTQ question above.
19. Does the name 2-butene indicate which of the stereoisomers above is being referred to? 20. To indicate the difference between alkenes that have 2 substituents across a double bond, use the prefix trans (across the double bond relative to each other) or cis (same side of the double bond t each other). Thus, the correct IUPAC names for the compounds above are cis-2-butene and trans butene. 21. Add cis or trans where needed to the IUPAC names in the alkene CTQ question above.
Organic Chemistry: A Guided Inquiry
2nd Edition
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Andrei Straumanis
Chapter6: Alkanes & Alkenes
Section: Chapter Questions
Problem 21E
Related questions
Question
Answer number 19, 20, and 21.
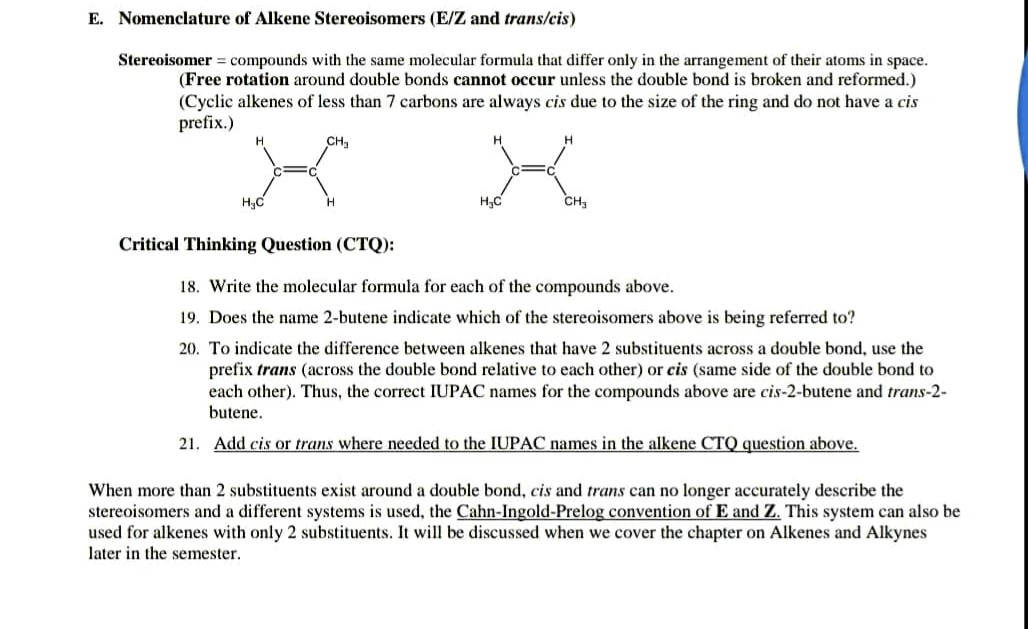
Transcribed Image Text:E. Nomenclature of Alkene Stereoisomers (E/Z and trans/cis)
Stereoisomer = compounds with the same molecular formula that differ only in the arrangement of their atoms in space.
(Free rotation around double bonds cannot occur unless the double bond is broken and reformed.)
(Cyclic alkenes of less than 7 carbons are always cis due to the size of the ring and do not have a cis
prefix.)
CH,
H.
H;C
CH,
Critical Thinking Question (CTQ):
18. Write the molecular formula for each of the compounds above.
19. Does the name 2-butene indicate which of the stereoisomers above is being referred to?
20. To indicate the difference between alkenes that have 2 substituents across a double bond, use the
prefix trans (across the double bond relative to each other) or cis (same side of the double bond to
each other). Thus, the correct IUPAC names for the compounds above are cis-2-butene and trans-2-
butene.
21. Add cis or trans where needed to the IUPAC names in the alkene CTQ question above.
When more than 2 substituents exist around a double bond, cis and trans can no longer accurately describe the
stereoisomers and a different systems is used, the Cahn-Ingold-Prelog convention of E and Z. This system can also be
used for alkenes with only 2 substituents. It will be discussed when we cover the chapter on Alkenes and Alkynes
later in the semester.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning