1A 8A H 2A 3A 4A 5A 6A 7A He BCNOF N Li Be 1B 2B AI Si P S CI Ar Na Mg 3B 48 58 6B 7B e K Ca Sc Ti V Cr Mn Fe Co Ni Cu Zn Ga Ge As Se Br Kr Rb Sr V 2r Nb Mo Te Ru Rh Pd Ag Ca In Sn Sb Te Xe CS Ba La H Ta w Re Os Ir Pt Au Hg T Pb Bi Po At Rn Fr Ra Ac Rf Ha Ce Pr Nd Pm Sm Eu 6d Tb Dy Ho Er Im Yb Lu Th Pa U Np Pu Am Cm Bk C Es Fm Md No Lr Amonatomic ion with a charge of -2 has an electronic configuration of ls22s22p3s^3p6 This ion is a(n) What is the chemical symbol of the noble gas this ion is isoelectronic with? What is the formula of the ion?
1A 8A H 2A 3A 4A 5A 6A 7A He BCNOF N Li Be 1B 2B AI Si P S CI Ar Na Mg 3B 48 58 6B 7B e K Ca Sc Ti V Cr Mn Fe Co Ni Cu Zn Ga Ge As Se Br Kr Rb Sr V 2r Nb Mo Te Ru Rh Pd Ag Ca In Sn Sb Te Xe CS Ba La H Ta w Re Os Ir Pt Au Hg T Pb Bi Po At Rn Fr Ra Ac Rf Ha Ce Pr Nd Pm Sm Eu 6d Tb Dy Ho Er Im Yb Lu Th Pa U Np Pu Am Cm Bk C Es Fm Md No Lr Amonatomic ion with a charge of -2 has an electronic configuration of ls22s22p3s^3p6 This ion is a(n) What is the chemical symbol of the noble gas this ion is isoelectronic with? What is the formula of the ion?
Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter2: Atoms, Molecules, And Ions
Section: Chapter Questions
Problem 85QAP
Related questions
Question
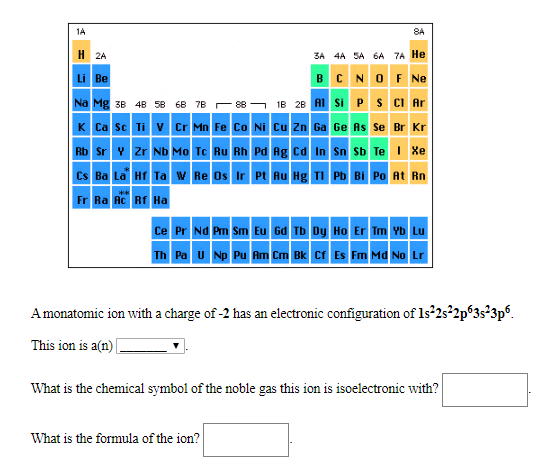
Transcribed Image Text:1A
8A
H 2A
3A 4A 5A 6A 7A He
BCNOF N
Li Be
1B 2B AI Si P S CI Ar
Na Mg 3B 48 58 6B 7B e
K Ca Sc Ti V Cr Mn Fe Co Ni Cu Zn Ga Ge As Se Br Kr
Rb Sr V 2r Nb Mo Te Ru Rh Pd Ag Ca In Sn Sb Te Xe
CS Ba La H Ta w Re Os Ir Pt Au Hg T Pb Bi Po At Rn
Fr Ra Ac Rf Ha
Ce Pr Nd Pm Sm Eu 6d Tb Dy Ho Er Im Yb Lu
Th Pa U Np Pu Am Cm Bk C Es Fm Md No Lr
Amonatomic ion with a charge of -2 has an electronic configuration of ls22s22p3s^3p6
This ion is a(n)
What is the chemical symbol of the noble gas this ion is isoelectronic with?
What is the formula of the ion?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps

Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning