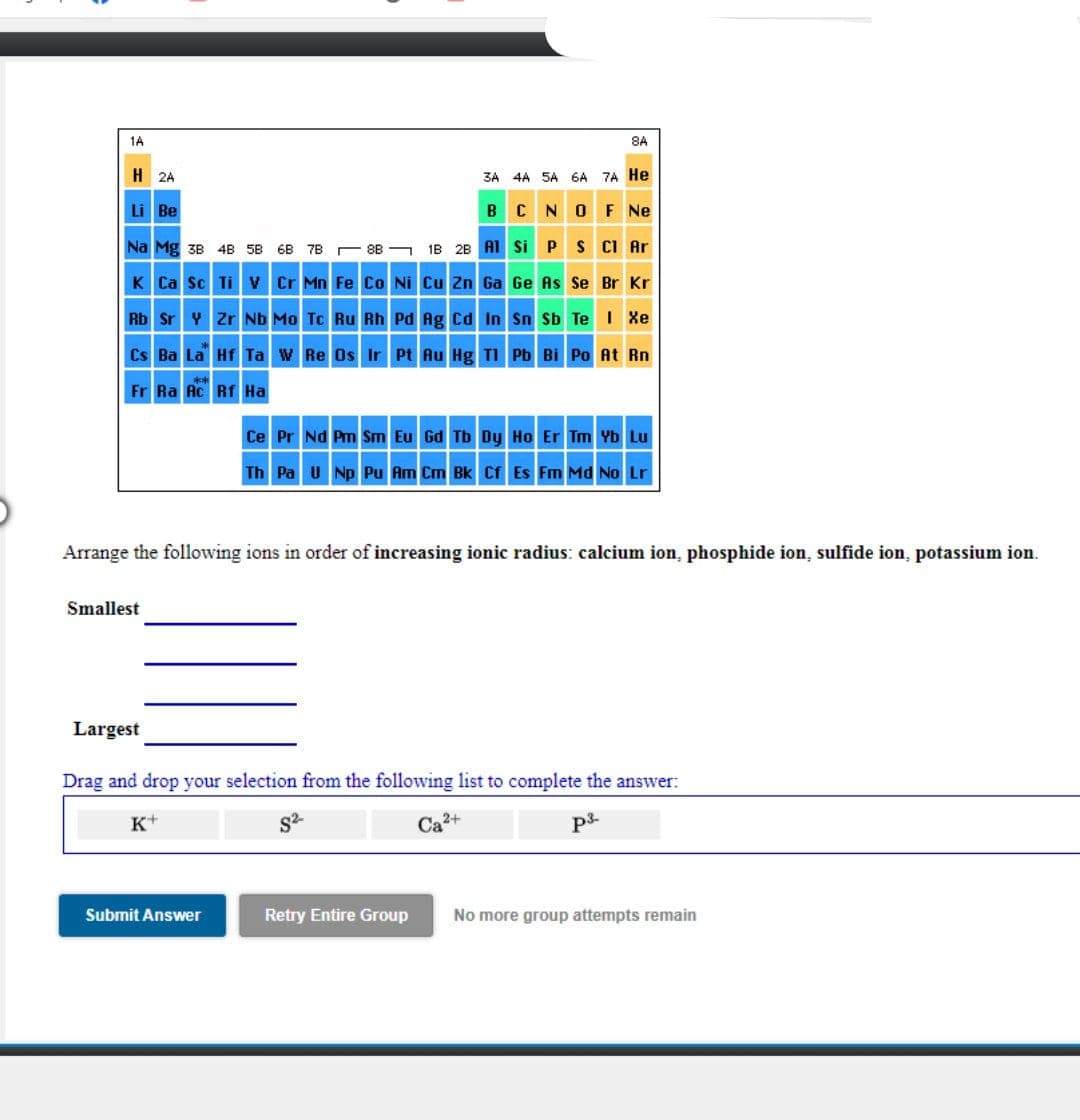
1A 8A H 2A 3A 4A SA 6A 7A He Li Be BCNOF Ne Na Mg 38 4B 5B 6B 7B 8B - 1B 2B AI Si P S CI Ar K Ca Sc Tiv Cr Mn Fe Co Ni Cu Zn Ga Ge As Se Br Kr Rb Sr Y Zr Nb Mo Tc Ru Rh Pd Ag Cd In Sn sb Te Xe Cs Ba La Hf Ta w Re Os Ir Pt Au Hg TI Pb Bi Po At Rn Fr Ra Ac Rf Ha Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho Er Tm Yb Lu Th Pa u Np Pu Am Cm Bk Cr Es Fm Md No Lr Arrange the following ions in order of increasing ionic radius: calcium ion, phosphide ion, sulfide ion, potassium ion. Smallest Largest Drag and drop your selection from the following list to complete the answer: K+ S2- Ca3+ Submit Answer Retry Entire Group No more group attempts remain
States of Matter
The substance that constitutes everything in the universe is known as matter. Matter comprises atoms which in turn are composed of electrons, protons, and neutrons. Different atoms combine together to give rise to molecules that act as a foundation for all kinds of substances. There are five states of matter based on their energies of attraction, namely solid, liquid, gases, plasma, and BEC (Bose-Einstein condensates).
Chemical Reactions and Equations
When a chemical species is transformed into another chemical species it is said to have undergone a chemical reaction. It consists of breaking existing bonds and forming new bonds by changing the position of electrons. These reactions are best explained using a chemical equation.
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images









