1A 8A H 2A 3A 4A SA 6A 7A He Li Be BCNOFNe 1B 2B AISi P SCI Ar Na Mg 3B 4B 58 6B 7B 88 K Ca Sc Ti V Cr Mn Fe Co Ni Cu Zn Ga Ge As Se Br Kr Rb Sr Zr Nb Mo Tc Ru Rh Pd Rg Cd In Sn Sb Te Xe CS Ba La Hr Ta w Re Os Ir Pt Au Hg T Pb Bi Po At Rn Fr Ra Ac Rf Ha Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho Er Tm Yb Lu Th Pa U Np Pu Am Cm Bk Cf Es Fm Ma No Lr (1) What is the valence electron configuration for the arsenic atom? (2) What is the valence electron configuration for the carbon atom?
1A 8A H 2A 3A 4A SA 6A 7A He Li Be BCNOFNe 1B 2B AISi P SCI Ar Na Mg 3B 4B 58 6B 7B 88 K Ca Sc Ti V Cr Mn Fe Co Ni Cu Zn Ga Ge As Se Br Kr Rb Sr Zr Nb Mo Tc Ru Rh Pd Rg Cd In Sn Sb Te Xe CS Ba La Hr Ta w Re Os Ir Pt Au Hg T Pb Bi Po At Rn Fr Ra Ac Rf Ha Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho Er Tm Yb Lu Th Pa U Np Pu Am Cm Bk Cf Es Fm Ma No Lr (1) What is the valence electron configuration for the arsenic atom? (2) What is the valence electron configuration for the carbon atom?
Chemistry: An Atoms First Approach
2nd Edition
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Steven S. Zumdahl, Susan A. Zumdahl
Chapter1: Chemical Foundations
Section: Chapter Questions
Problem 1RQ: Define and e xplain the differences between the following terms. a. law and theory b. theory and...
Related questions
Question
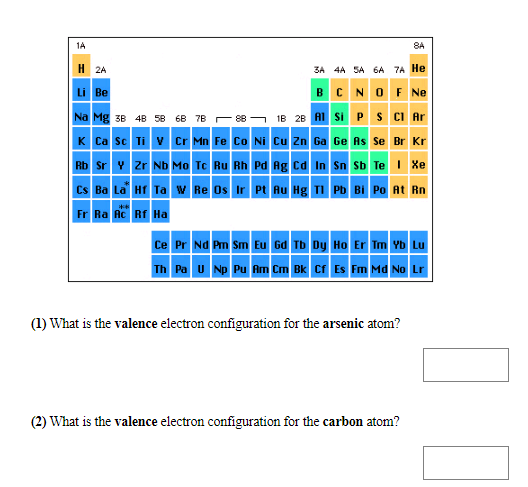
Transcribed Image Text:1A
8A
H 2A
3A 4A SA 6A 7A He
Li Be
BCNOFNe
1B 2B AISi P SCI Ar
Na Mg 3B 4B 58 6B 7B 88
K Ca Sc Ti V Cr Mn Fe Co Ni Cu Zn Ga Ge As Se Br Kr
Rb Sr Zr Nb Mo Tc Ru Rh Pd Rg Cd In Sn Sb Te Xe
CS Ba La Hr Ta w Re Os Ir Pt Au Hg T Pb Bi Po At Rn
Fr Ra Ac Rf Ha
Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho Er Tm Yb Lu
Th Pa U Np Pu Am Cm Bk Cf Es Fm Ma No Lr
(1) What is the valence electron configuration for the arsenic atom?
(2) What is the valence electron configuration for the carbon atom?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps

Recommended textbooks for you

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning