Na Mg Al Si CI Ar 19 20 21 25 22 23 Ti 24 26 27 28 29 30 31 32 33 34 35 36 K Ca Sc V Cr Mn Fe Co Ni Cu Zn Ga Ge As Se Br Kr 37 38 Sr 39 40 41 42 43 44 45 46 47 48 49 50 51 52 53 54 Rb Zr Nb Mo Tc Ru Rh Pd Ag Cd In Sn Sb Te Xe 55 56 Ba 75 Re W 57 72 73 Hf Ta 74 76 Os 77 78 Ir Pt 79 80 81 TI 82 Pb 83 Bi 84 Po 85 At 86 Rn Cs La Au 87 Fr 8 89 Ra 104 105 106 107 108 109 110 111 112 113 114 115 116 117 118 Rg Ac Rf Db Sg Bh Hs Mt Ds Cn Uut FI Uup Ly Uus Uuo 58 59 60 Nd 61 Pm 63 Sm 62 64 65 66 67 68 Er 69 70 71 冰 Ce Pr Eu Gd Tb Dy Ho Tm Yb Lu 92 93 94 99 100 101 102 103 90 Th 91 95 96 97 98 Pa Np Pu Am Cm Bk Cf Es Fm Md No Lr Use the Periodic Table to determine which of the following lists consists of elements that have the MOST similar chemical properties. A Mg, Al, and Si K, Ca, and Ga C Mg, Ca, and Ba K, AI, and Ni ©2021 Illuminate Education TM, Inc.
Na Mg Al Si CI Ar 19 20 21 25 22 23 Ti 24 26 27 28 29 30 31 32 33 34 35 36 K Ca Sc V Cr Mn Fe Co Ni Cu Zn Ga Ge As Se Br Kr 37 38 Sr 39 40 41 42 43 44 45 46 47 48 49 50 51 52 53 54 Rb Zr Nb Mo Tc Ru Rh Pd Ag Cd In Sn Sb Te Xe 55 56 Ba 75 Re W 57 72 73 Hf Ta 74 76 Os 77 78 Ir Pt 79 80 81 TI 82 Pb 83 Bi 84 Po 85 At 86 Rn Cs La Au 87 Fr 8 89 Ra 104 105 106 107 108 109 110 111 112 113 114 115 116 117 118 Rg Ac Rf Db Sg Bh Hs Mt Ds Cn Uut FI Uup Ly Uus Uuo 58 59 60 Nd 61 Pm 63 Sm 62 64 65 66 67 68 Er 69 70 71 冰 Ce Pr Eu Gd Tb Dy Ho Tm Yb Lu 92 93 94 99 100 101 102 103 90 Th 91 95 96 97 98 Pa Np Pu Am Cm Bk Cf Es Fm Md No Lr Use the Periodic Table to determine which of the following lists consists of elements that have the MOST similar chemical properties. A Mg, Al, and Si K, Ca, and Ga C Mg, Ca, and Ba K, AI, and Ni ©2021 Illuminate Education TM, Inc.
General Chemistry - Standalone book (MindTap Course List)
11th Edition
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Chapter2: Atoms, Molescules, And Ions
Section: Chapter Questions
Problem 2.59QP
Related questions
Question
This is NOT graded!
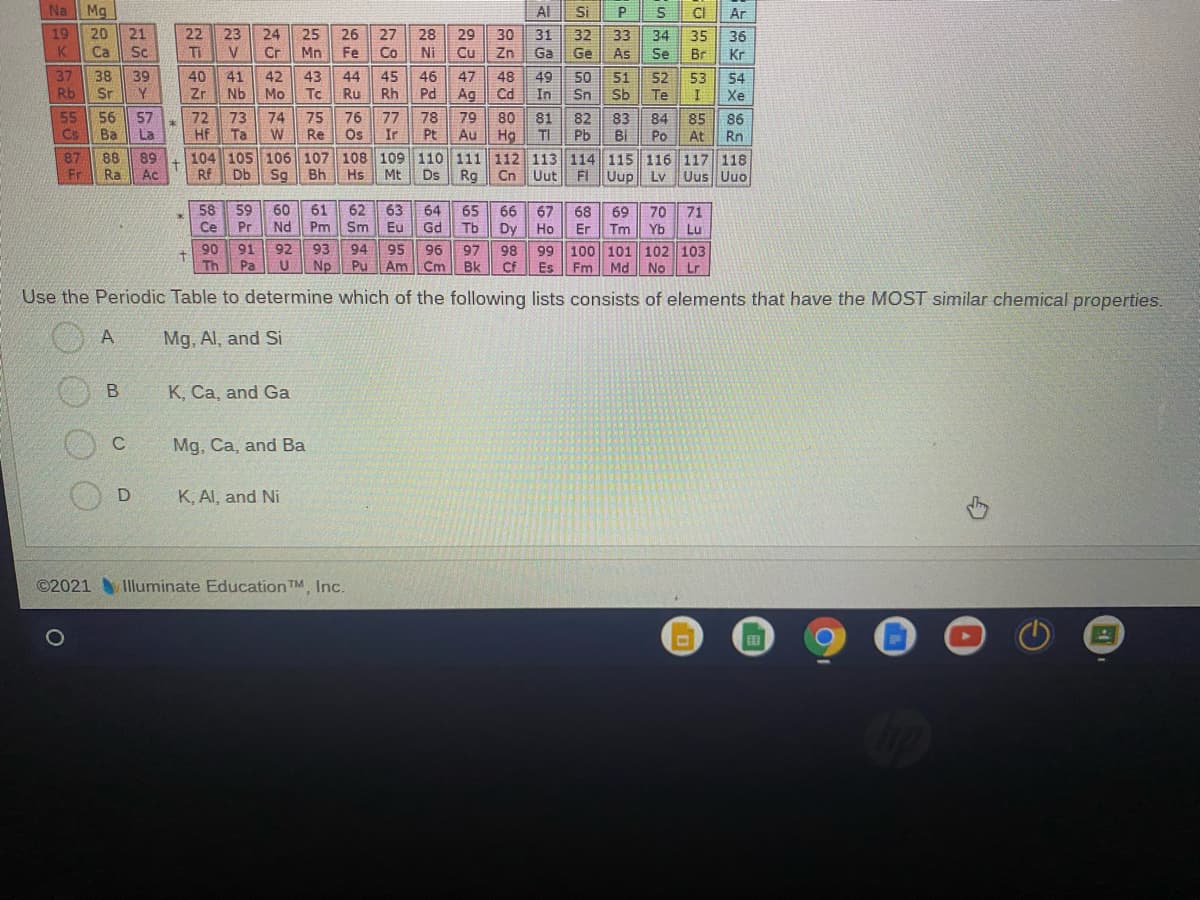
Transcribed Image Text:Na Mg
Al
Si
P
CI
Ar
19
20
K
Ca
21
Sc
22
Ti
23
24
25
26
27
28
29
30
31
32
33
34
35
36
V
Cr
Mn
Fe
Co
Ni
Cu
Zn
Ga
Ge
As
Se
Br
Kr
37
Rb
38 39
Sr
40
41
42
43
44
45
46
47
48
49
50
51
52
53
54
Zr
Nb
Mo
Tc
Ru
Rh
Pd
Ag
Cd
In
Sn
Sb
Te
Xe
55
Cs
56
Ba
57
La
72
73
Hf
Ta
74
75
Re
76
77
78
Ir
Pt
79
80
81
TI
82
Pb
83
84
Po
85
At
86
Rn
Os
Hg
104 105 106 107 108 109 110 111 112 113 114 115 116 117 118
Rg
Au
BI
87
Fr
88
89
Ra
Ac
Rf
Db
Sq
Bh
Hs
Mt
Ds
Cn Uut
FI
Uup
Ly
Uus Uuo
58
59
66
60
Nd
61
Pm
62
Sm
63
64
65
Tb
67
Dy Ho
68
Er
69
Tm
70
Yb
71
Ce
Pr
Eu
Gd
Lu
90
91
92
93
94
95
99 100 101 102 103
96
97
Pu Am Cm
98
4.
Th
Fm
Pa
Np
Bk
CF
Es
Md No Lr
Use the Periodic Table to determine which of the following lists consists of elements that have the MOST similar chemical properties.
A
Mg, Al, and Si
K, Ca, and Ga
C
Mg, Ca, and Ba
K, Al, and Ni
©2021 Illuminate Education TM, Inc.
B.
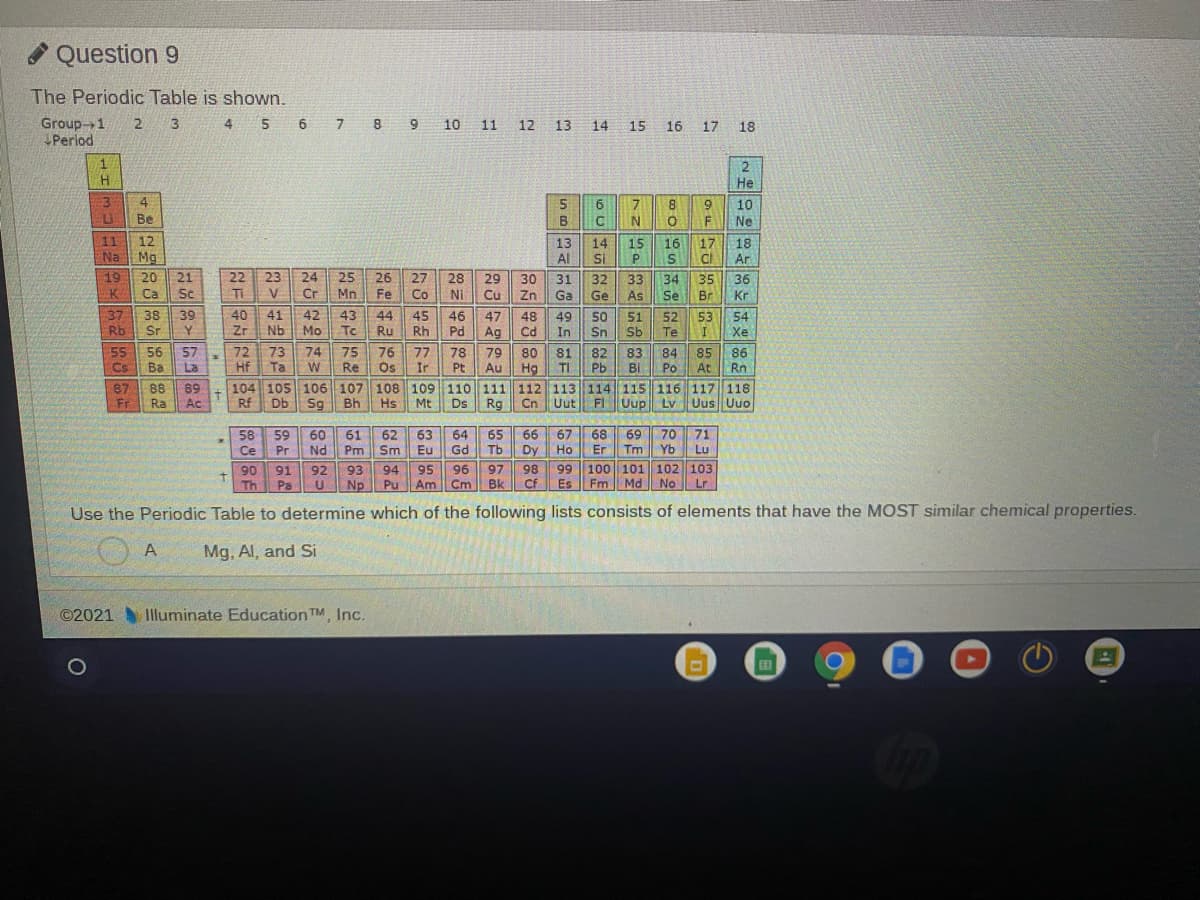
Transcribed Image Text:* Question 9
The Periodic Table is shown.
6 7 8 9
Group-1
Perlod
2.
3
4.
10 11 12 13 14
15
16 17 18
1
He
4
6.
10
Be
B
N.
F
Ne
11
12
13
14
Si
15
16
17
CI
18
Na Mg
Al
Ar
19
20
21
22
23
24
25
26
27
28
29
30
31
32
33
34
35
36
K
Ca
Sc
TI
V
Cr
Mn
Fe
Co
Ni
Cu
Zn
Ga
Ge
As
Se
Br
Kr
37
Rb Sr
39
40
Zr
41
Nb
42
Mo
43
Tc
38
44
Ru
45
Rh
46
Pd
47
48
Cd
49
In
50
Sn
51
Sb
52
Te
53
54
Xe
Ag
55
56
57
La
72
Hf
73
Ta
74
75
Re
76
Os
77
Ir
78
Pt
79
Au
80
81
TI
82
Pb
83
84
85
At
86
Rn
Cs
Ba
W
Hg
Bi Po
87
Fr
104 105 106 107 108 109 110 111 112 113 114 115 116 117 118
Ds Rg
88
89
Ac
Ra
Rf
Db
Sg
Bh
Hs
Mt
Cn
Uut
FI
Uup
Uup Lv Uus Uuo
66
65
67
Tb
Dy
68
70
Tm Yb
69
71
Lu
58
59
60
Nd
61
Pm
62
Sm
63
Eu
64
Gd
Ce
Pr
Но
Er
92
98
99 100 101 102 103
94
Np Pu
90
91
93
95
96
97
Th
Pa
Am
Cm
Bk
Cf
Es
Fm Md No Lr
Use the Periodic Table to determine which of the following lists consists of elements that have the MOST similar chemical properties.
A
Mg. Al, and Si
©2021 Illuminate Education TM, Inc.
田
72
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning