2 3 4 5 Data Table 1: Mass, Volume, and Density of Water for Trials 1-5. (Be sure to include the correctly abbreviated units.) Trial Total Mass of Water 1 57.33 57.3369 62.3899 67.486g 72.535 535g 77.734g Average Density of Water Total Volume of Water 5.11ml 10.15ml 15.36ml 20.5l ml 25.99 ml Density of Water _1. g/m/ 0.981g/ml 0.9911g/ml 0.9870g/m²/ 0.989 9854 g/m1 0.9929g/m1
2 3 4 5 Data Table 1: Mass, Volume, and Density of Water for Trials 1-5. (Be sure to include the correctly abbreviated units.) Trial Total Mass of Water 1 57.33 57.3369 62.3899 67.486g 72.535 535g 77.734g Average Density of Water Total Volume of Water 5.11ml 10.15ml 15.36ml 20.5l ml 25.99 ml Density of Water _1. g/m/ 0.981g/ml 0.9911g/ml 0.9870g/m²/ 0.989 9854 g/m1 0.9929g/m1
Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter1: The Nature Of Chemistry
Section: Chapter Questions
Problem 127QRT
Related questions
Question

Transcribed Image Text:2
3
5
Data Table 1: Mass, Volume, and Density of Water for Trials 1-5. (Be sure to include
the correctly abbreviated units.)
Trial
1 57.33
Total Mass of Water Total Volume of Water
57.3369
62.3899
67.486g
72.535g
77.7349
4 72.5
Average Density of Water
5.11ml
10.15ml
15.36ml
20.5l ml
25.99 ml
1. g/ml
Density of Water
0.981g/ml
0.9911g/ml
0.9870g/m/
0.9854 g/m1
0.9929ğıml
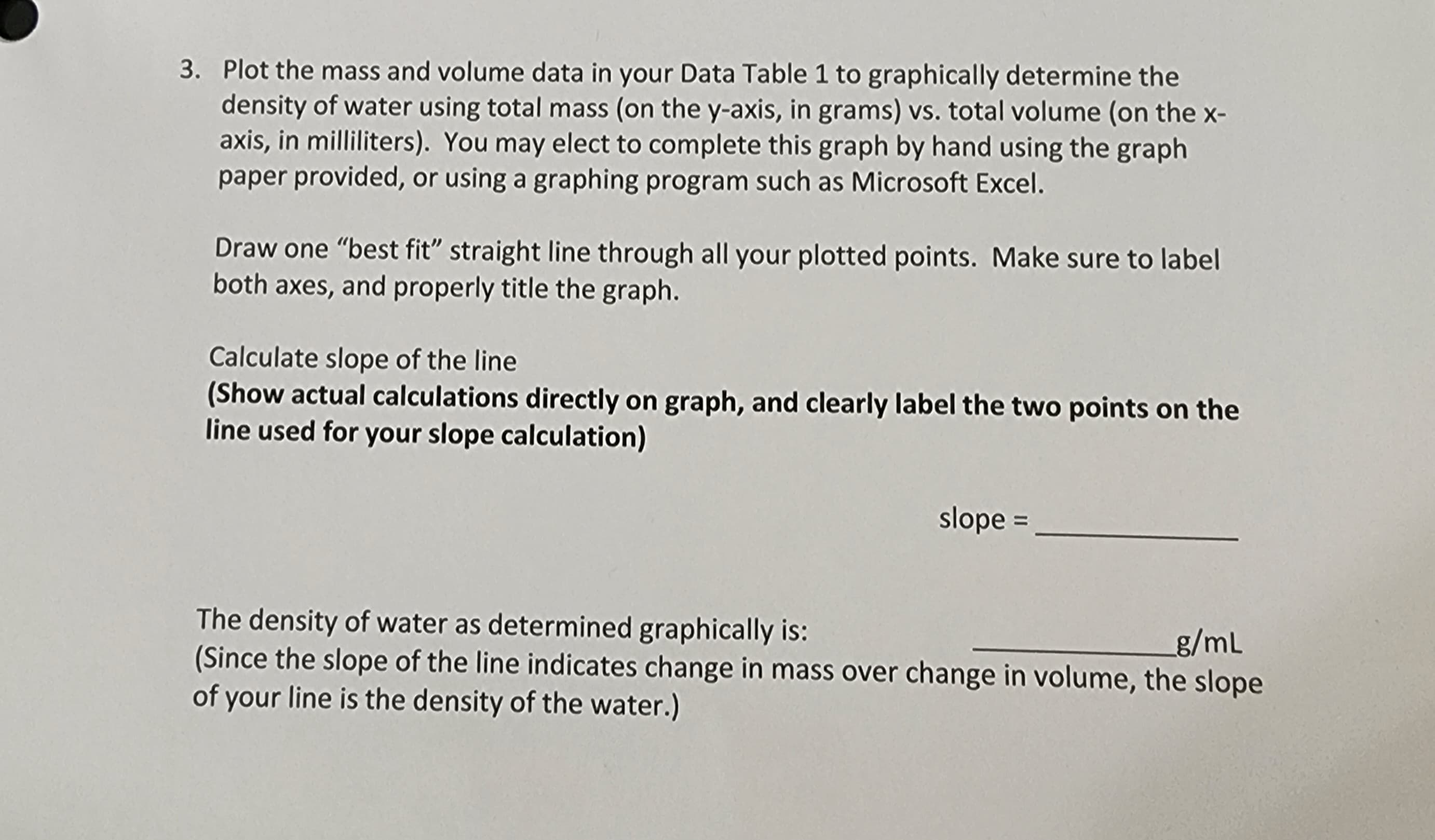
Transcribed Image Text:3. Plot the mass and volume data in your Data Table 1 to graphically determine the
density of water using total mass (on the y-axis, in grams) vs. total volume (on the x-
axis, in milliliters). You may elect to complete this graph by hand using the graph
paper provided, or using a graphing program such as Microsoft Excel.
Draw one "best fit" straight line through all your plotted points. Make sure to label
both axes, and properly title the graph.
Calculate slope of the line
(Show actual calculations directly on graph, and clearly label the two points on the
line used for your slope calculation)
slope =______
The density of water as determined graphically is:
_g/mL
(Since the slope of the line indicates change in mass over change in volume, the slope
of your line is the density of the water.)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning


Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning
