2. Consider the compound below. In the figure, each dot represents one atom (color coded/numbered). The lines indicate that two atoms are bound together; each line may represent an ionic, single covalent, double covalent, or triple covalent bond. Carbon Hydrogen Oxygen 1 1 Potassium 1 1 a. Draw a complete, correct Lewis structure. Include any resonance necessary. b. Give the number of electron groups on each atom. c. For any atom with hybridized orbitals, indicate the hybridization. d. Indicate the total number of o and Tt covalent bonds in the molecule.
2. Consider the compound below. In the figure, each dot represents one atom (color coded/numbered). The lines indicate that two atoms are bound together; each line may represent an ionic, single covalent, double covalent, or triple covalent bond. Carbon Hydrogen Oxygen 1 1 Potassium 1 1 a. Draw a complete, correct Lewis structure. Include any resonance necessary. b. Give the number of electron groups on each atom. c. For any atom with hybridized orbitals, indicate the hybridization. d. Indicate the total number of o and Tt covalent bonds in the molecule.
Chapter9: Covalent Bonding: Orbitals
Section: Chapter Questions
Problem 68AE: Aspartame is an artificial sweetener marketed under the name Nutra-Sweet. A partial Lewis structure...
Related questions
Question
Help me please
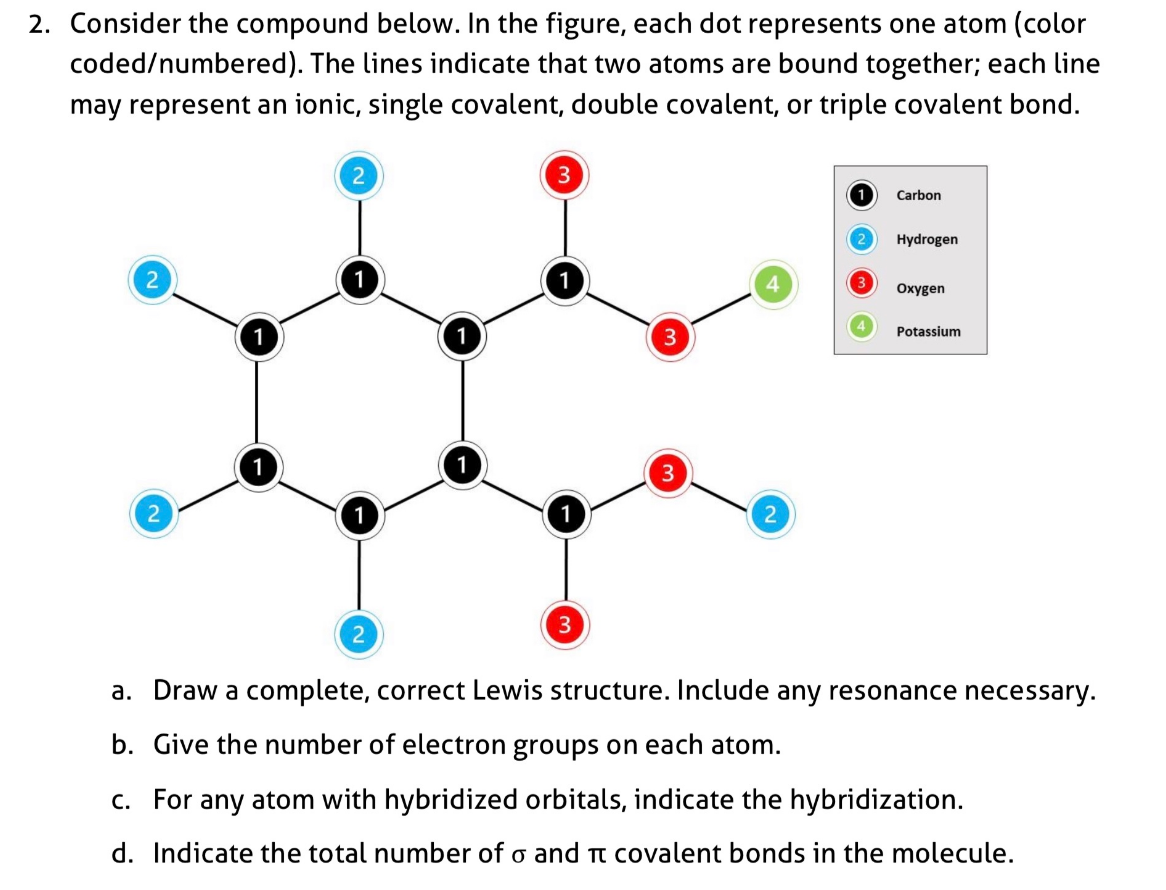
Transcribed Image Text:2. Consider the compound below. In the figure, each dot represents one atom (color
coded/numbered). The lines indicate that two atoms are bound together; each line
may represent an ionic, single covalent, double covalent, or triple covalent bond.
Carbon
Hydrogen
Oxygen
Potassium
1
1
1
1
a. Draw a complete, correct Lewis structure. Include any resonance necessary.
b. Give the number of electron groups on each atom.
c. For any atom with hybridized orbitals, indicate the hybridization.
d. Indicate the total number of o and Tt covalent bonds in the molecule.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning