2. The SOHIO ammoxidation process of propyte ne to acrytonitrile is given by the reaction: C3 HG (9) + Oa C9) + NH3 (9) – Ca Hg N(9) + Hạ0 (g9) kg Reaction was done at 430°C and 0.126 atm. If it is desired to produce 2400 of hr m3 is used? consider 80% conversion on hr acrylonitrile, how much propylent in propylene.
2. The SOHIO ammoxidation process of propyte ne to acrytonitrile is given by the reaction: C3 HG (9) + Oa C9) + NH3 (9) – Ca Hg N(9) + Hạ0 (g9) kg Reaction was done at 430°C and 0.126 atm. If it is desired to produce 2400 of hr m3 is used? consider 80% conversion on hr acrylonitrile, how much propylent in propylene.
Chapter5: Gases
Section: Chapter Questions
Problem 74E: Urea (H2NCONH2) is used extensively as a nitrogen source in fertilizers. It is produced commercially...
Related questions
Question
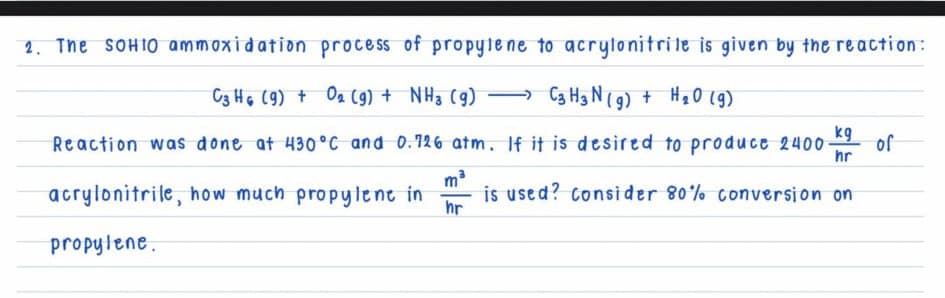
Transcribed Image Text:2. The SOHIO ammoxidation process of propyle ne to acrytonitrile is given by the reaction:
C3 HG (9) + Oa (9) + NH3 (g)
Ca Hg N(9) + Hạ0 (g9)
kg
Reaction was done at 430°C and 0.126 atm. If it is desired to produce 2400
of
hr
acrylonitrile, how much propylent in
is used? consider 80% conversion on
hr
propylene.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning