23 Which one below is true for the reaction stages during photocatalytic activity of TiO,? * O2 Adsorption CB Reduction hv > Eg H2O Eg Degradation of organic compounds/ microorganisms/ pollutants CO2 + H2O ht ht VB •OH -HOK ´Oxidation Adsorption O All of them. Superoxide and hydroxyl radicals react
23 Which one below is true for the reaction stages during photocatalytic activity of TiO,? * O2 Adsorption CB Reduction hv > Eg H2O Eg Degradation of organic compounds/ microorganisms/ pollutants CO2 + H2O ht ht VB •OH -HOK ´Oxidation Adsorption O All of them. Superoxide and hydroxyl radicals react
Organic Chemistry
8th Edition
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Chapter21: Benzene And The Concept Of Aromaticity
Section: Chapter Questions
Problem 21.43P: Following is an equation for iodination of toluene. This reaction does not take place. All that...
Related questions
Question
23
Transcribed Image Text:23
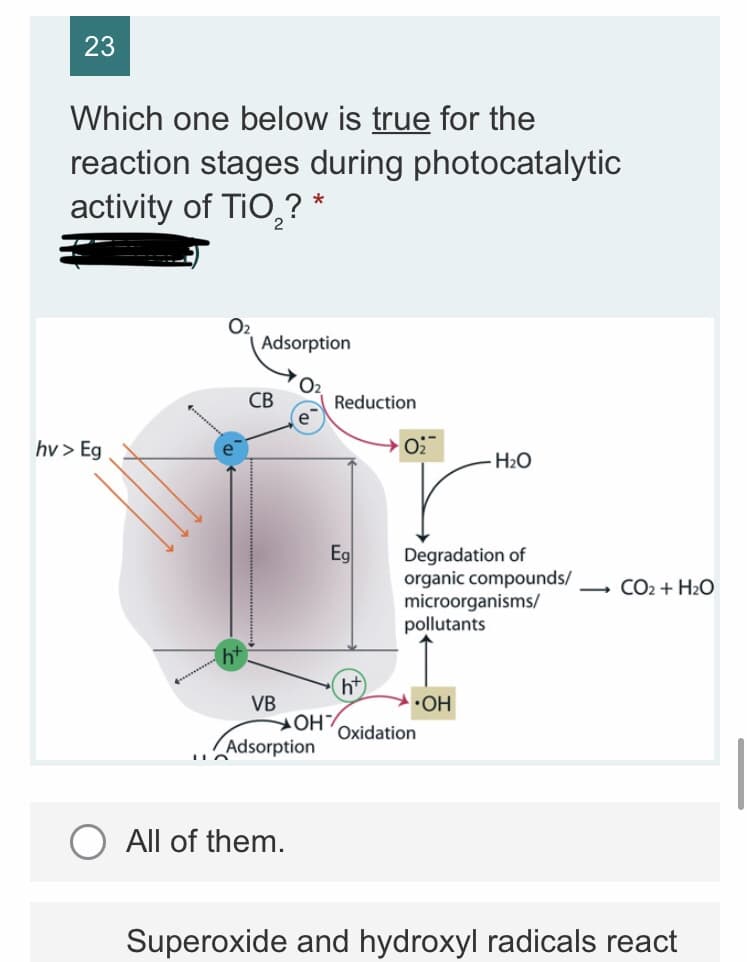
Which one below is true for the
reaction stages during photocatalytic
activity of TiO,? *
O2
Adsorption
СВ
Reduction
hv> Eg
H2O
Eg
Degradation of
organic compounds/
microorganisms/
pollutants
CO2 + H2O
ht
VB
•OH
Oxidation
Adsorption
All of them.
Superoxide and hydroxyl radicals react

Transcribed Image Text:VB
HO-
HOK
Oxidation
Adsorption
All of them.
Superoxide and hydroxyl radicals react
with organics, microorganisms, and
pollutants adsorbed on the surface of
TiO, resulting in hydroxylation,
oxidation, and finally mineralization to
2'
carbon dioxide and water.
Photo-induced holes in the valence
band diffuse to the surface and likely
react with adsorbed water and hydroxyl
ions to form reactive hydroxyl radicals,
which are strong oxidizing agents.
Photo-induced electrons in the
conduction band typically reductively
react with adsorbed oxygen in air to
produce reactive superoxide radicals.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,