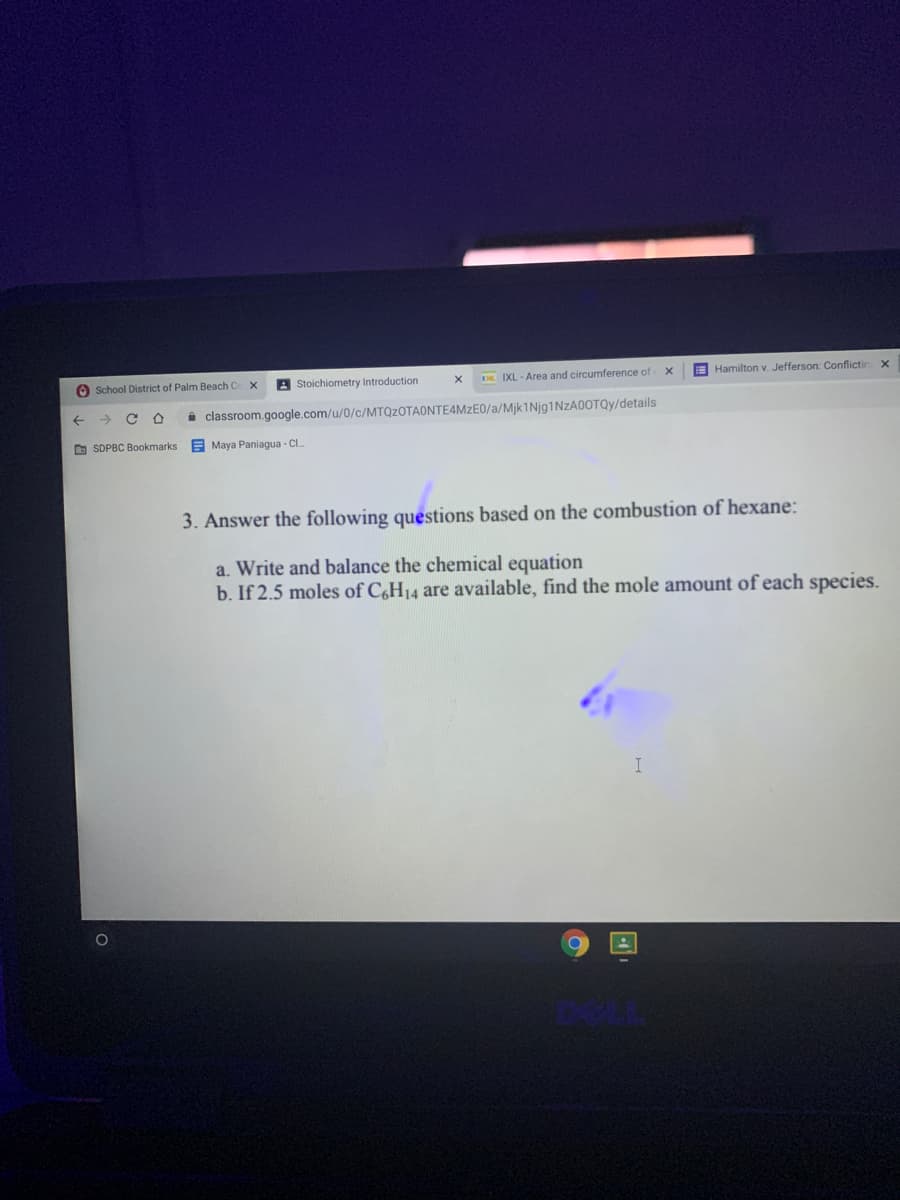
3. Answer the following questions based on the combustion of hexane: a. Write and balance the chemical equation b. If 2.5 moles of C6H14 are available, find the mole amount of each species.
3. Answer the following questions based on the combustion of hexane: a. Write and balance the chemical equation b. If 2.5 moles of C6H14 are available, find the mole amount of each species.
Chemistry: An Atoms First Approach
2nd Edition
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Steven S. Zumdahl, Susan A. Zumdahl
Chapter8: Gases
Section: Chapter Questions
Problem 155CP: Methane (CH4) gas flows into a combustion chamber at a rate of 200. L/min at 1.50 atm and ambient...
Related questions
Question
Transcribed Image Text:O School District of Palm Beach C
A Stoichiometry Introduction
L IXL - Area and circumference of
E Hamilton v. Jefferson: Conflictin x
->
classroom.google.com/u/0/c/MTQZOTAONTE4MZE0/a/Mjk1Njg1NzA00TQy/details
O SDPBC Bookmarks
E Maya Paniagua - CI.
3. Answer the following questions based on the combustion of hexane:
a. Write and balance the chemical equation
b. If 2.5 moles of C,H14 are available, find the mole amount of each species.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning
