3. Seven analyses for the phosphorus content of a fertilizer resulted in 16.2, 17.5, 15.4, 15.9, 16., 16.3, and 17.1%. Find (a) the standard deviation, S, and (b) the 95% confidence interval for the true value, H. 4. The following five values were obtained for the wt % of an organic acid in a sample: 30.3, 31.1,
3. Seven analyses for the phosphorus content of a fertilizer resulted in 16.2, 17.5, 15.4, 15.9, 16., 16.3, and 17.1%. Find (a) the standard deviation, S, and (b) the 95% confidence interval for the true value, H. 4. The following five values were obtained for the wt % of an organic acid in a sample: 30.3, 31.1,
Principles of Instrumental Analysis
7th Edition
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
ChapterA1: Evaluation Of Analytical Data
Section: Chapter Questions
Problem A1.8QAP
Related questions
Question
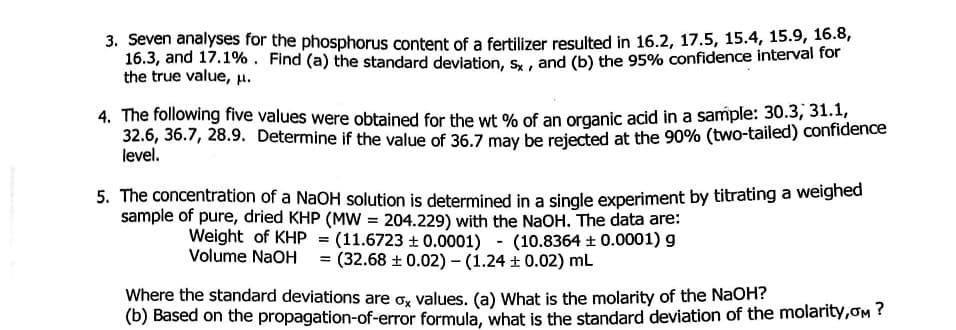
Transcribed Image Text:3. Seven analyses for the phosphorus content of a fertilizer resulted in 16.2, 17.5, 15.4, 15.9, 16.8,
16.3, and 17.1%. Find (a) the standard deviation, s, , and (b) the 95% confidence interval for
the true value, H.
4. The following five values were obtained for the wt % of an organic acid in a sample: 30.3, 31.1,
32.6, 36.7, 28.9. Determine if the value of 36.7 may be rejected at the 90% (two-tailed) confidence
level.
5. The concentration of a NaOH solution is determined in a single experiment by titrating a weighed
sample of pure, dried KHP (MW = 204.229) with the NaOH. The data are:
Weight of KHP = (11.6723 + 0.0001) - (10.8364 ±0.0001) g
Volume NaOH = (32.68 + 0.02) - (1.24 + 0.02) mL
Where the standard deviations are o, values. (a) What is the molarity of the NaOH?
(b) Based on the propagation-of-error formula, what is the standard deviation of the molarity,oM ?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning


Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning
