3. Which of the following relates to the phenomenon of electrons occupying orbitals at the same energy sublevel singly before any orbital is occupied by two electrons? Aufbau Principle Electron Configuration Hund's Rule Orbital Diagram Pauli Exclusion Principle а. d. b. е. С. f. Quantum Mechanical Theory
3. Which of the following relates to the phenomenon of electrons occupying orbitals at the same energy sublevel singly before any orbital is occupied by two electrons? Aufbau Principle Electron Configuration Hund's Rule Orbital Diagram Pauli Exclusion Principle а. d. b. е. С. f. Quantum Mechanical Theory
Physical Chemistry
2nd Edition
ISBN:9781133958437
Author:Ball, David W. (david Warren), BAER, Tomas
Publisher:Ball, David W. (david Warren), BAER, Tomas
Chapter11: Quantum Mechanics: Model Systems And The Hydrogen Atom
Section: Chapter Questions
Problem 11.69E: Construct an energy level diagram showing all orbitals for the hydrogen atom up to n=5, labeling...
Related questions
Question
100%
Pls help ASAP.
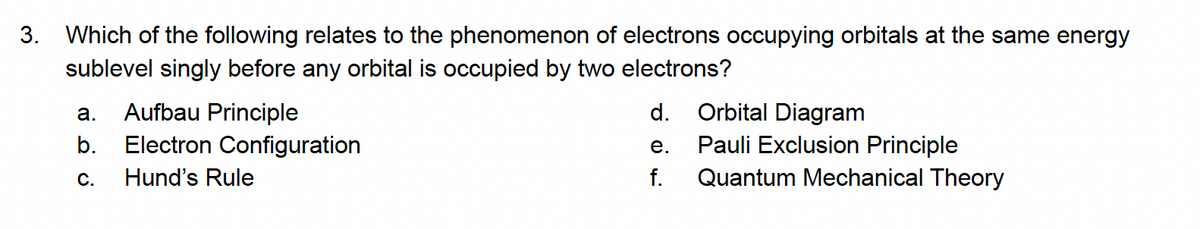
Transcribed Image Text:3. Which of the following relates to the phenomenon of electrons occupying orbitals at the same energy
sublevel singly before any orbital is occupied by two electrons?
Aufbau Principle
d.
Orbital Diagram
а.
Pauli Exclusion Principle
Quantum Mechanical Theory
b.
Electron Configuration
е.
С.
Hund's Rule
f.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Recommended textbooks for you

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning