4. Using this formula Cu = Au x Cstd. Astd Compute for the concentration of the uric acid of the patient,Convert your answer to mmol/L(S.I.) by multiplying it to 0.059 C = concentration A = absorbance U = unknown/sample STD = standard Absorbance of the standard: 0.109 Concentration of the standard: 0.55mg/dL Absorbance of the unknown: 0.241
4. Using this formula Cu = Au x Cstd. Astd Compute for the concentration of the uric acid of the patient,Convert your answer to mmol/L(S.I.) by multiplying it to 0.059 C = concentration A = absorbance U = unknown/sample STD = standard Absorbance of the standard: 0.109 Concentration of the standard: 0.55mg/dL Absorbance of the unknown: 0.241
Chapter16: Applications Of Neutralization Titrations
Section: Chapter Questions
Problem 16.18QAP
Related questions
Question
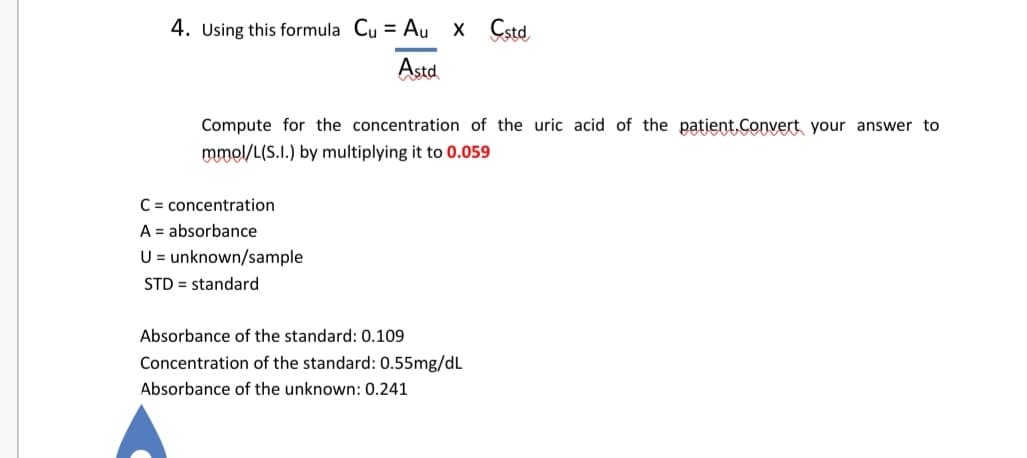
Transcribed Image Text:4. Using this formula Cu = Au x Cstd
Astd
Compute for the concentration of the uric acid of the patient.Convert your answer to
mmol/L(S.I.) by multiplying it to 0.059
C = concentration
A = absorbance
U = unknown/sample
STD = standard
Absorbance of the standard: 0.109
Concentration of the standard: 0.55mg/dL
Absorbance of the unknown: 0.241
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 1 images

Recommended textbooks for you



Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning



Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning