The following data were obtained for 1.00 cm samples of a particular chemical. What is the concentration of a 1.00 cm sample with an absorbance of 0.60 according to the graph? absorbance 0.8 0.7 y 1.4x-0.01.. 0.6 0.5 0.4 0.3 0.2 0.1 0.1 0.2 0.3 0.4 0.5 0.6 Concentration Round to 2 decimal places, do not include units. Answer: Absorbance
The following data were obtained for 1.00 cm samples of a particular chemical. What is the concentration of a 1.00 cm sample with an absorbance of 0.60 according to the graph? absorbance 0.8 0.7 y 1.4x-0.01.. 0.6 0.5 0.4 0.3 0.2 0.1 0.1 0.2 0.3 0.4 0.5 0.6 Concentration Round to 2 decimal places, do not include units. Answer: Absorbance
Chapter25: Instruments For Optical Spectrometry
Section: Chapter Questions
Problem 25.6QAP
Related questions
Question
Learning guidelines
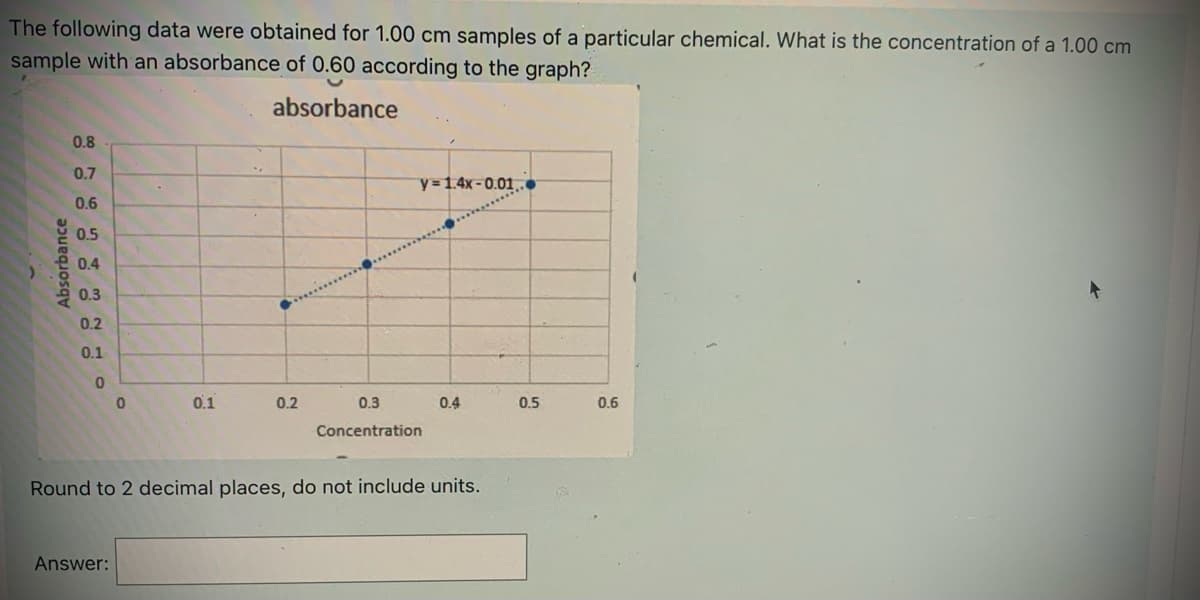
Transcribed Image Text:The following data were obtained for 1.00 cm samples of a particular chemical. What is the concentration of a 1.00 cm
sample with an absorbance of 0.60 according to the graph?
absorbance
0.8
0.7
y=1.4x-0.01..
0.6
0.5
0.4
0.3
0.2
0.1
0.1
0.2
0.3
0.4
0.5
0.6
Concentration
Round to 2 decimal places, do not include units.
Answer:
Absorbance
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Recommended textbooks for you


Macroscale and Microscale Organic Experiments
Chemistry
ISBN:
9781305577190
Author:
Kenneth L. Williamson, Katherine M. Masters
Publisher:
Brooks Cole

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning


Macroscale and Microscale Organic Experiments
Chemistry
ISBN:
9781305577190
Author:
Kenneth L. Williamson, Katherine M. Masters
Publisher:
Brooks Cole

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning


Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning