6. Copper(1) iodide, Cul, is not stable enough to last long in storage, so it is generally made just prior to use. In can be prepared from copper sulphate and hydriodic acid by the following reaction: 2 Cuso,(s) + 4 HI(aq) - a) If 10.4 grams of CuSO, is used, calculate the number of grams of HI needed and the ---> 2 Cul(s) + 2 H,So,(aq) + L,(s) number of grams of each of the products that are produced. Show that the mass data are in accordance with the law of conservation of mass in chemical reactions. b) If 10.4 grams of copper(I) iodide were isolated from the reaction above, what is the percent yield of the reaction?
6. Copper(1) iodide, Cul, is not stable enough to last long in storage, so it is generally made just prior to use. In can be prepared from copper sulphate and hydriodic acid by the following reaction: 2 Cuso,(s) + 4 HI(aq) - a) If 10.4 grams of CuSO, is used, calculate the number of grams of HI needed and the ---> 2 Cul(s) + 2 H,So,(aq) + L,(s) number of grams of each of the products that are produced. Show that the mass data are in accordance with the law of conservation of mass in chemical reactions. b) If 10.4 grams of copper(I) iodide were isolated from the reaction above, what is the percent yield of the reaction?
Introductory Chemistry: A Foundation
9th Edition
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Donald J. DeCoste
Chapter9: Chemical Quantities
Section: Chapter Questions
Problem 40QAP
Related questions
Question
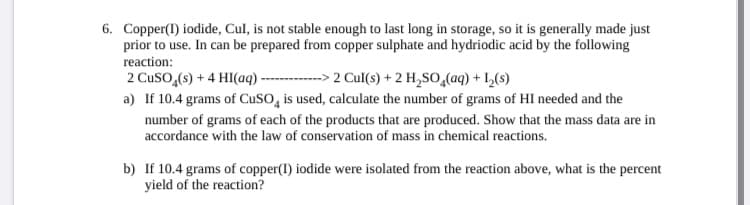
Transcribed Image Text:6. Copper(1) iodide, Cul, is not stable enough to last long in storage, so it is generally made just
prior to use. In can be prepared from copper sulphate and hydriodic acid by the following
reaction:
2 Cuso,(s) + 4 HI(aq) -
a) If 10.4 grams of CuSO, is used, calculate the number of grams of HI needed and the
---> 2 Cul(s) + 2 H,So,(aq) + L,(s)
number of grams of each of the products that are produced. Show that the mass data are in
accordance with the law of conservation of mass in chemical reactions.
b) If 10.4 grams of copper(I) iodide were isolated from the reaction above, what is the percent
yield of the reaction?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

World of Chemistry
Chemistry
ISBN:
9780618562763
Author:
Steven S. Zumdahl
Publisher:
Houghton Mifflin College Div

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

World of Chemistry
Chemistry
ISBN:
9780618562763
Author:
Steven S. Zumdahl
Publisher:
Houghton Mifflin College Div

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemical Principles in the Laboratory
Chemistry
ISBN:
9781305264434
Author:
Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:
Brooks Cole

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning