60. Consider the reaction. CO(g) + Cl2(g) — COCl2(g) Kp = 1.49 × 108 at 373.2 K An equilibrium mixture contains a CO and Cl₂ partial pressure of 0.1702 torr. Calculate the equilibrium partial pressures of the product.
60. Consider the reaction. CO(g) + Cl2(g) — COCl2(g) Kp = 1.49 × 108 at 373.2 K An equilibrium mixture contains a CO and Cl₂ partial pressure of 0.1702 torr. Calculate the equilibrium partial pressures of the product.
Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter12: Gaseous Chemical Equilibrium
Section: Chapter Questions
Problem 70QAP: For the reaction C(s)+CO2(g)2CO(g) K=168 at 1273 K. If one starts with 0.3 atm of CO2 and 12.0 g of...
Related questions
Question
If neccessary *USE QUADRATIC equation: Onlinemschool.com
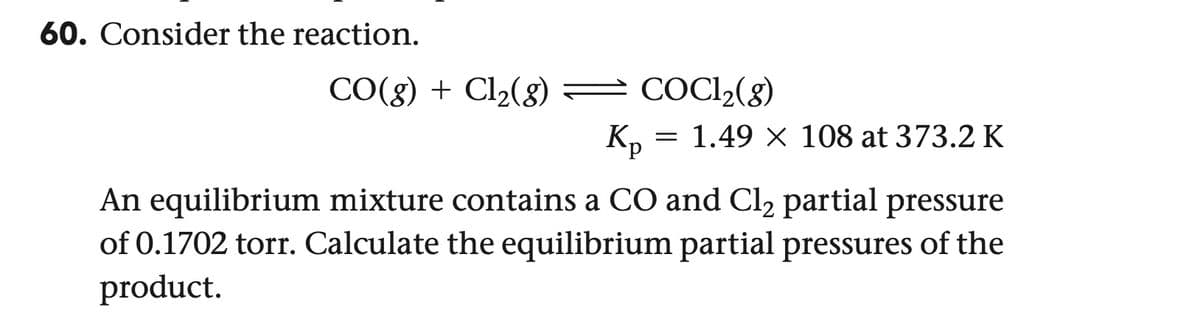
Transcribed Image Text:60. Consider the reaction.
CO(g) + Cl2(g) — COCl2(g)
Kp = 1.49 × 108 at 373.2 K
An equilibrium mixture contains a CO and Cl₂ partial pressure
of 0.1702 torr. Calculate the equilibrium partial pressures of the
product.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 1 images

Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning