Introductory Chemistry: A Foundation
9th Edition
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Donald J. DeCoste
Chapter17: Equilibrium
Section: Chapter Questions
Problem 3QAP: How do chemists envision reactions taking place in terms of the collision model for reactions? Give...
Related questions
Question
I need help with only questions 7-9
(Not honor class)
(Not grading)
Transcribed Image Text:Types of Chemical Reactions Practice - 1322616 @
Tools Add-ons Help
Last edit was seconds ago
text
BIUA .
Arial
14
三
1
2 .
3
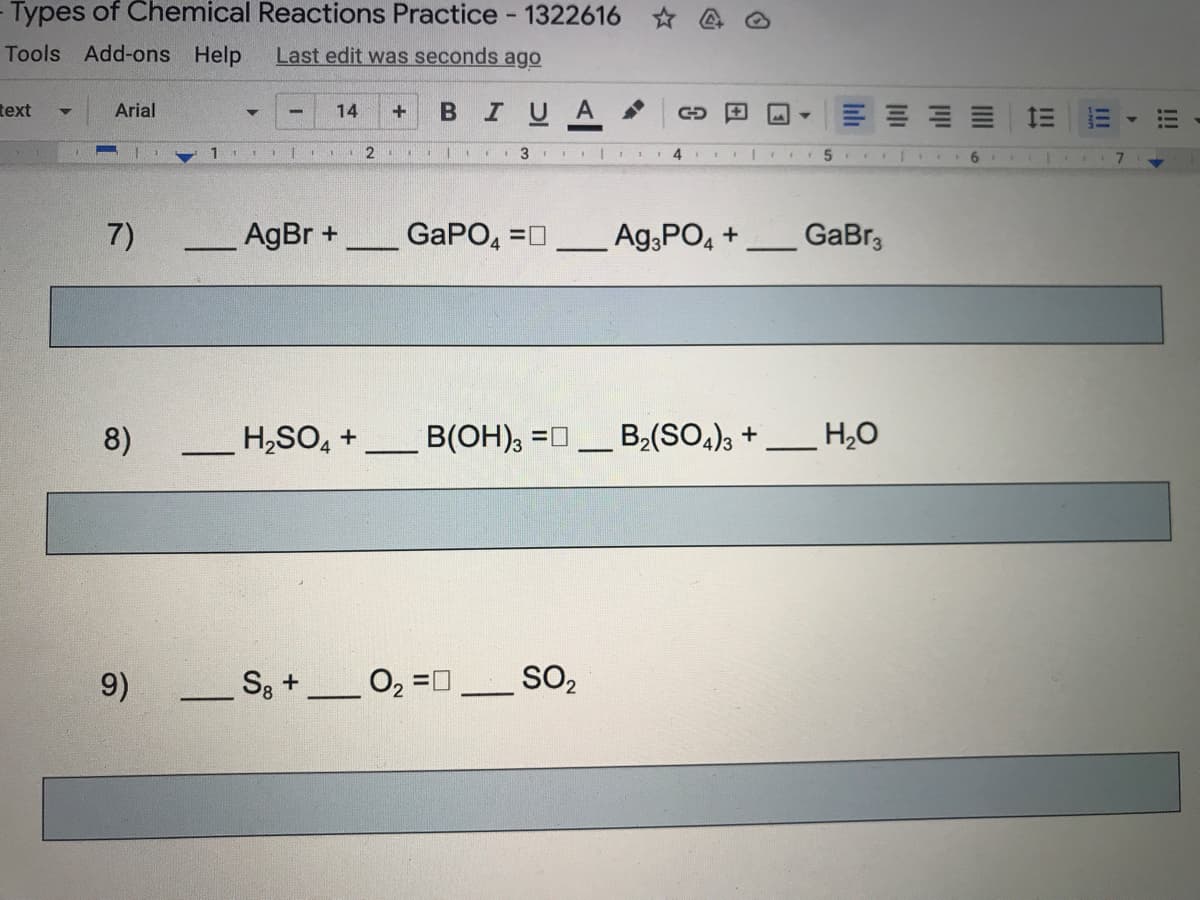
7)
AgBr +
_GaPO, =n
Ag,PO, +
GaBr3
4
8)
H,SO, +
B(OH), =0
B2(SO,)3 +
H20
-
9)
S3 +
SO2
!!!
II
II!
III
Transcribed Image Text:5
6 I
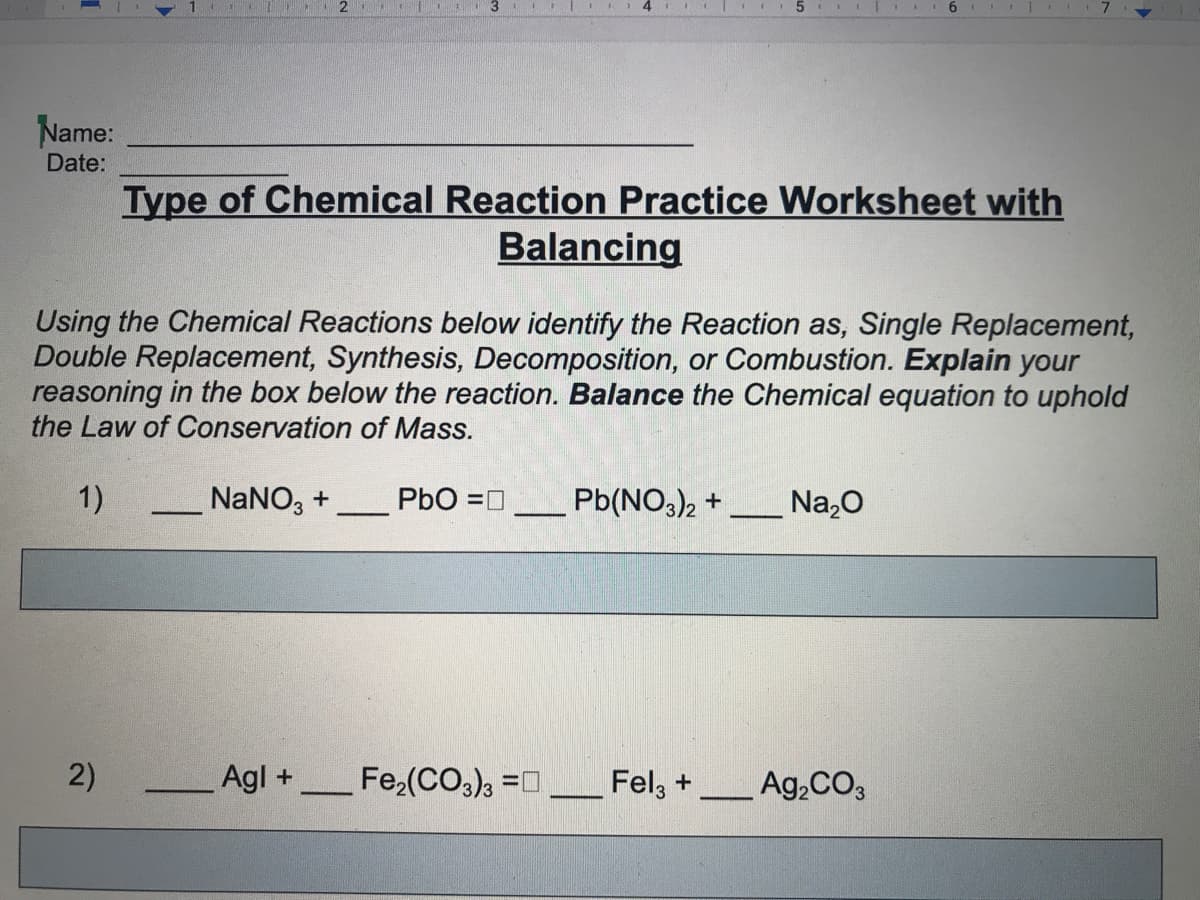
Name:
Date:
Type of Chemical Reaction Practice Worksheet with
Balancing
Using the Chemical Reactions below identify the Reaction as, Single Replacement,
Double Replacement, Synthesis, Decomposition, or Combustion. Explain your
reasoning in the box below the reaction. Balance the Chemical equation to uphold
the Law of Conservation of Mass.
1)
NaNO, +
PbO =0
Pb(NO,)2 +
Na,0
2)
Agl +
Fe,(CO), =0
Fel, +
Ag,CO3
%3D
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning