85.A solution of known concentration used to standardize another solution is: 1. Primary standard II. Secondary standard III. Dilute solution a. II, III d. I, II b. e. || C. I, II, III I
85.A solution of known concentration used to standardize another solution is: 1. Primary standard II. Secondary standard III. Dilute solution a. II, III d. I, II b. e. || C. I, II, III I
Introductory Chemistry: An Active Learning Approach
6th Edition
ISBN:9781305079250
Author:Mark S. Cracolice, Ed Peters
Publisher:Mark S. Cracolice, Ed Peters
Chapter16: Solutions
Section: Chapter Questions
Problem 14E
Related questions
Question
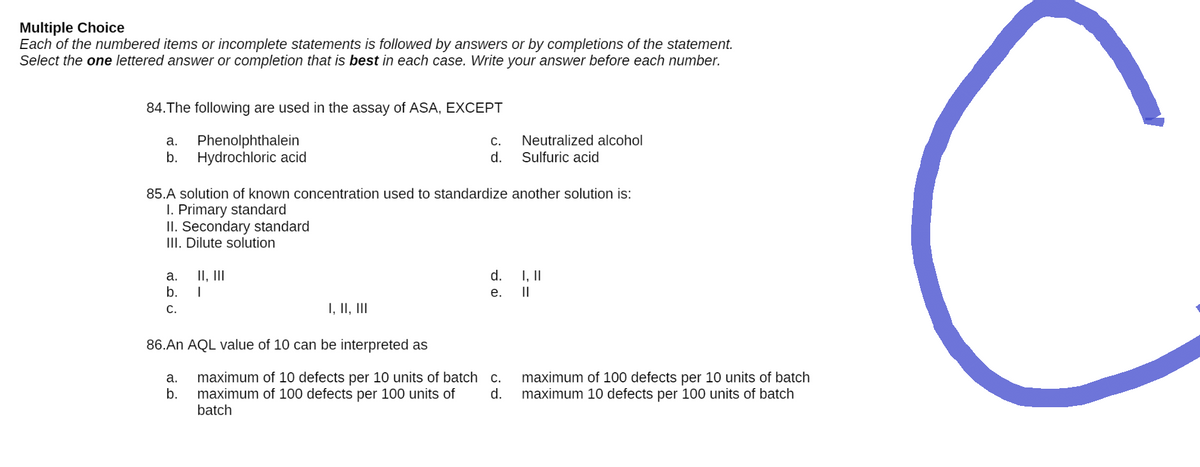
Transcribed Image Text:Multiple Choice
Each of the numbered items or incomplete statements is followed by answers or by completions of the statement.
Select the one lettered answer or completion that is best in each case. Write your answer before each number.
84. The following are used in the assay of ASA, EXCEPT
a.
Phenolphthalein
C.
Neutralized alcohol
Sulfuric acid
b. Hydrochloric acid
d.
85.A solution of known concentration used to standardize another solution is:
I. Primary standard
II. Secondary standard
III. Dilute solution
a. II, III
d.
I, II
b. I
e.
||
C.
I, II, III
86.An AQL value of 10 can be interpreted as
a.
c.
maximum of 10 defects per 10 units of batch
maximum of 100 defects per 100 units of
batch
maximum of 100 defects per 10 units of batch
maximum 10 defects per 100 units of batch
b.
d.
C
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning

