_C2H6 +_O2-→_CO2+ _H20 In the above equation, what will be the sum of its coefficients when it is balanced with the smallest possible whole number? A. 4 В. 8 С. 19 D. 38
For items 1-4 please refer to the problem posted on the picture. choose the correct answer correctly.
1. In the above equation, what will be the sum of its coefficients when it is balanced with the smallest possible whole number?
A. 4 B. 8 C. 19 D. 38
2. How many moles of O2 are required with one mole of ethane, C2H6?
A. 2.0 moles B. 4.0 moles
C. 3.5 moles D. 7.0 moles
3. How many moles of CO2 are formed when one mole of O2 is consumed?
A. 0.14 mol CO2 B. 0.57 mol CO2
C. 1.00 mol CO2 D. 4.00 mol CO2
4. How many moles of CO2 are formed when 5 moles of C2H6 are consumed?
A. 2.5 moles CO2 B. 5.0 moles CO2
C. 10.0 moles CO2 D. 20.0 moles CO2

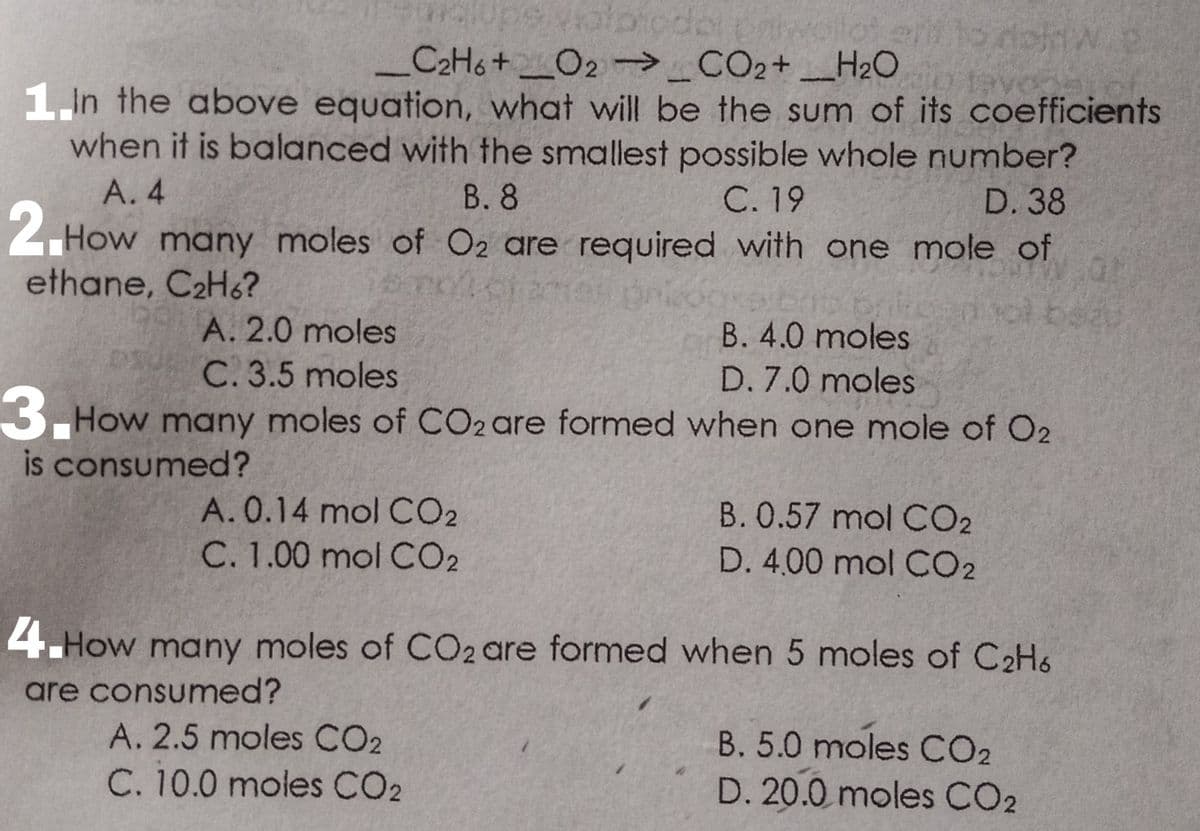
Trending now
This is a popular solution!
Step by step
Solved in 2 steps









