A 1.000 mL aliquot of a solution containing Cu²+ and Ni2+ is treated with 25.00 mL of a 0.03835 M EDTA solution. The solution is then back titrated with 0.02240 M Zn²+ solution at a pH of 5. A volume of 15.56 mL of the Zn2+ solution was needed to reach the xylenol orange end point. A 2.000 mL aliquot of the Cu²+ and Ni²+ solution is fed through an ion- exchange column that retains Ni²+. The Cu²+ that passed through the column is treated with 25.00 mL of 0.03835 M EDTA. This solution required 19.69 mL of 0.02240 M Zn²+ for back titration. The Ni²+ extracted from the column was treated witn 25.00 mL of 0.03835 M EDTA. How many milliliters of 0.02240 M Zn²+ is required for the back titration of the Ni²+ solution? volume: mL
A 1.000 mL aliquot of a solution containing Cu²+ and Ni2+ is treated with 25.00 mL of a 0.03835 M EDTA solution. The solution is then back titrated with 0.02240 M Zn²+ solution at a pH of 5. A volume of 15.56 mL of the Zn2+ solution was needed to reach the xylenol orange end point. A 2.000 mL aliquot of the Cu²+ and Ni²+ solution is fed through an ion- exchange column that retains Ni²+. The Cu²+ that passed through the column is treated with 25.00 mL of 0.03835 M EDTA. This solution required 19.69 mL of 0.02240 M Zn²+ for back titration. The Ni²+ extracted from the column was treated witn 25.00 mL of 0.03835 M EDTA. How many milliliters of 0.02240 M Zn²+ is required for the back titration of the Ni²+ solution? volume: mL
Chemistry & Chemical Reactivity
10th Edition
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter4: Stoichiometry: Quantitative Information About Chemical Reactions
Section4.9: Spectrophotometry
Problem 3.1ACP
Related questions
Question
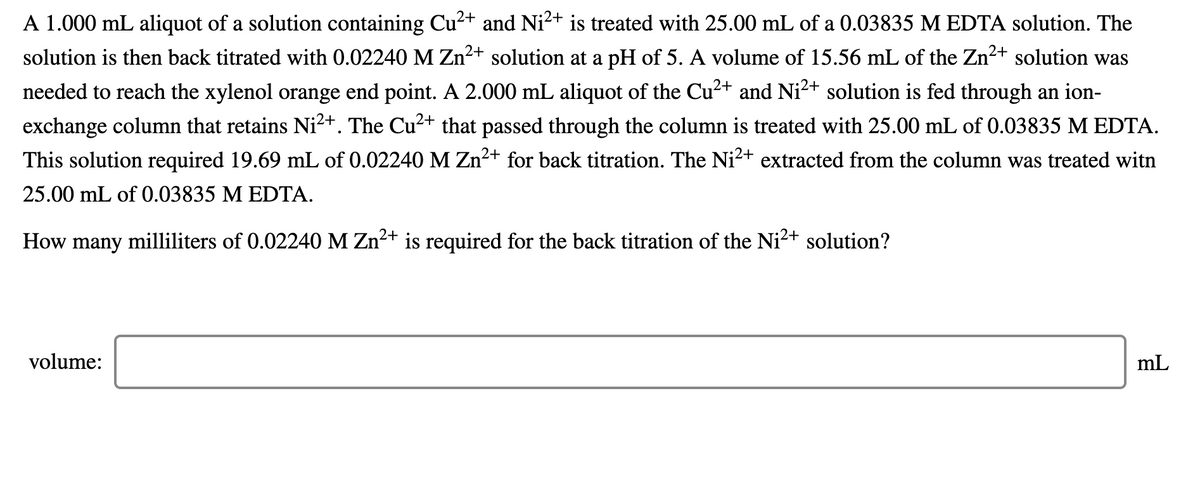
Transcribed Image Text:A 1.000 mL aliquot of a solution containing Cu²+ and Ni2+ is treated with 25.00 mL of a 0.03835 M EDTA solution. The
solution is then back titrated with 0.02240 M Zn2+ solution at a pH of 5. A volume of 15.56 mL of the Zn2+ solution was
needed to reach the xylenol orange end point. A 2.000 mL aliquot of the Cu2+ and Ni2+ solution is fed through an ion-
exchange column that retains Ni²+. The Cu²+ that passed through the column is treated with 25.00 mL of 0.03835 M EDTA.
This solution required 19.69 mL of 0.02240M Zn2+ for back titration. The Ni2+ extracted from the column was treated witn
25.00 mL of 0.03835 M EDTA.
How many milliliters of 0.02240 M Zn2+ is required for the back titration of the Ni2+ solution?
volume:
mL
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning



Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

