Principles of Instrumental Analysis
7th Edition
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Chapter33: Automated Methods Of Analysis
Section: Chapter Questions
Problem 33.8QAP
Related questions
Question
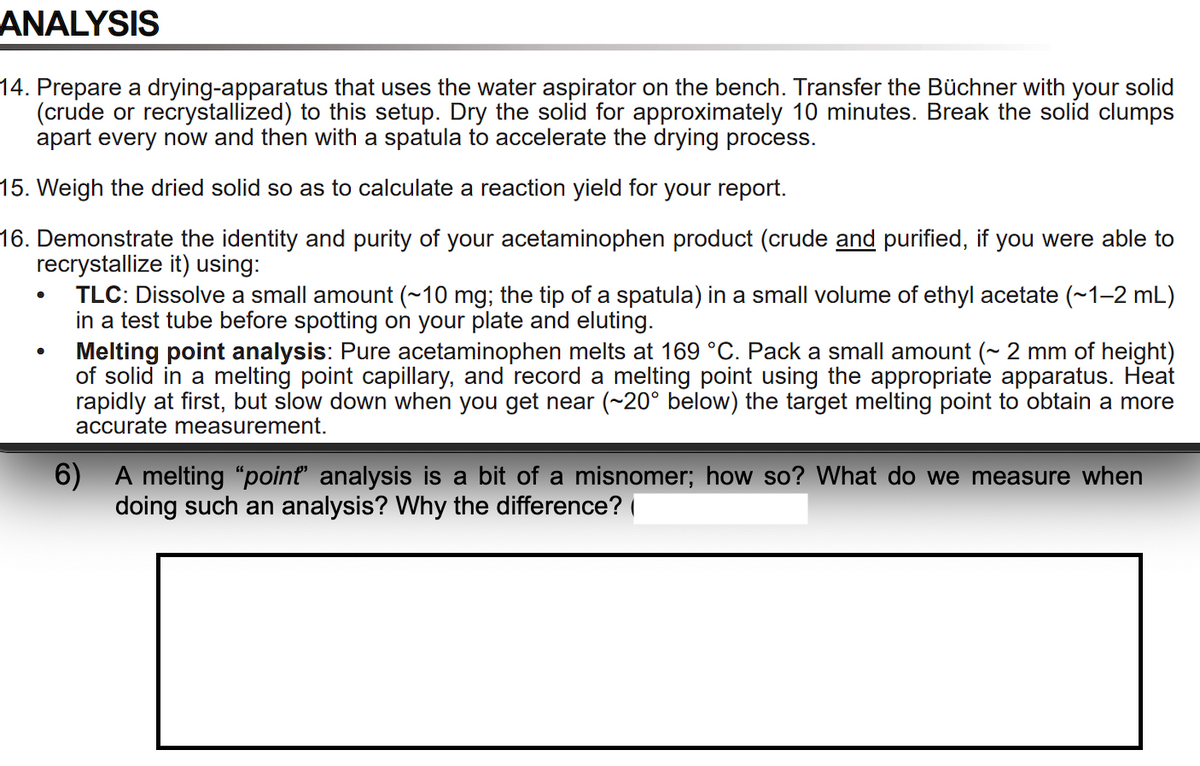
Transcribed Image Text:ANALYSIS
14. Prepare a drying-apparatus that uses the water aspirator on the bench. Transfer the Büchner with your solid
(crude or recrystallized) to this setup. Dry the solid for approximately 10 minutes. Break the solid clumps
apart every now and then with a spatula to accelerate the drying process.
15. Weigh the dried solid so as to calculate a reaction yield for your report.
16. Demonstrate the identity and purity of your acetaminophen product (crude and purified, if you were able to
recrystallize it) using:
TLC: Dissolve a small amount (~10 mg; the tip of a spatula) in a small volume of ethyl acetate (~1-2 mL)
in a test tube before spotting on your plate and eluting.
Melting point analysis: Pure acetaminophen melts at 169 °C. Pack a small amount (~ 2 mm of height)
of solid in a melting point capillary, and record a melting point using the appropriate apparatus. Heat
rapidly at first, but slow down when you get near (~20° below) the target melting point to obtain a more
accurate measurement.
6) A melting "point" analysis is a bit of a misnomer; how so? What do we measure when
doing such an analysis? Why the difference? |
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Recommended textbooks for you

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT

Macroscale and Microscale Organic Experiments
Chemistry
ISBN:
9781305577190
Author:
Kenneth L. Williamson, Katherine M. Masters
Publisher:
Brooks Cole

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT

Macroscale and Microscale Organic Experiments
Chemistry
ISBN:
9781305577190
Author:
Kenneth L. Williamson, Katherine M. Masters
Publisher:
Brooks Cole