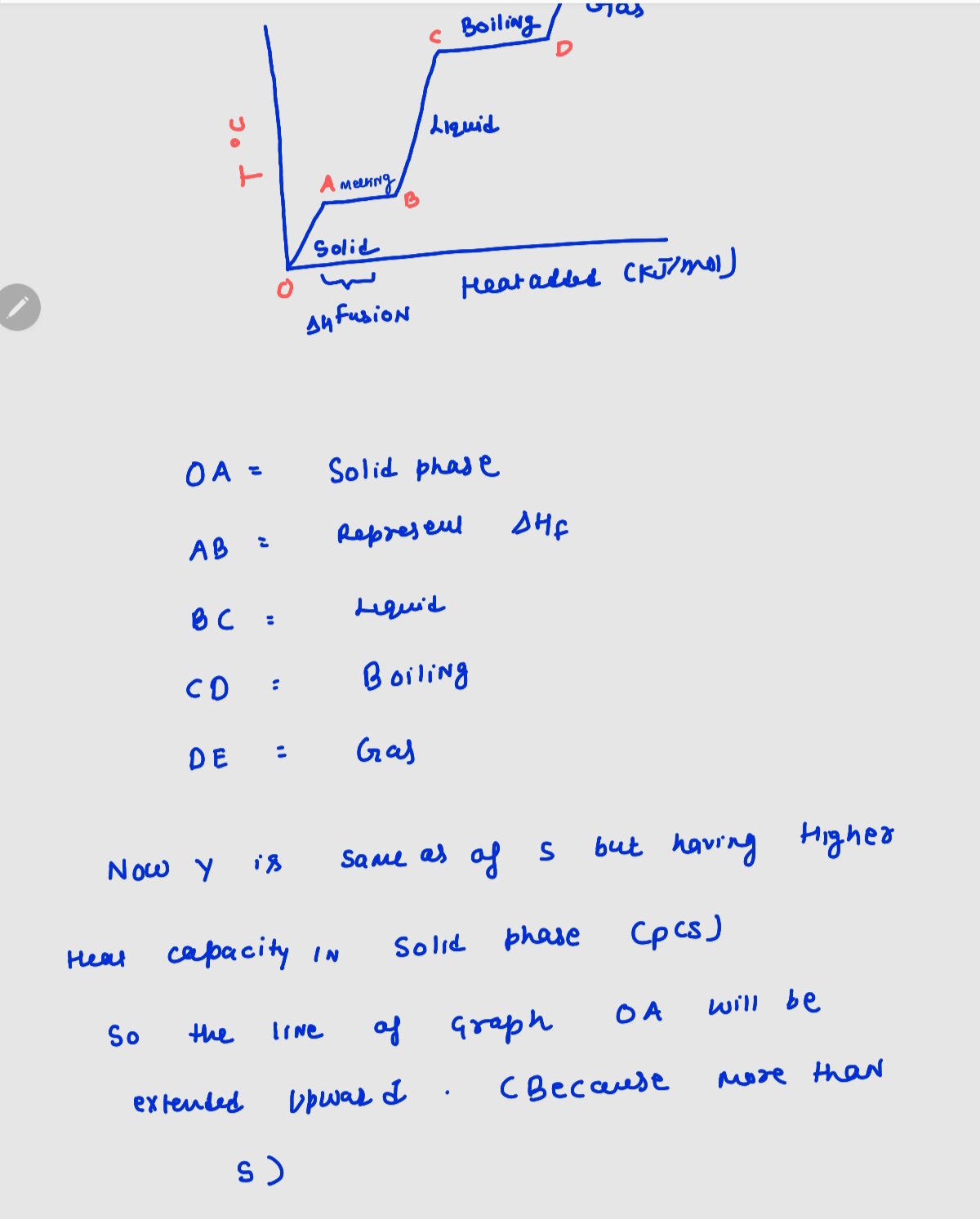
A pure sample of Substance S is put into an evacuated flask. The flask is then heated steadily and the temperature measured as time passes. The results are graphed below, in the middle (in green). Identical experiments are now run on Substance Y and Substance Z. Substance Y is just like S except that it has a higher heat capacity in the solid phase Cp(s). Substance Z is just like S except that it has a lower enthalpy of fusion AH. Select the graphs below, on the left and right, that show the results you expect for these new experiments. Substance Y (higher C(s)) (Drag the slider to choose an image) ¡ temperature (°C) Substance S added heat (kJ/mol) Substance Z (lower ΔΗ.) (Drag the slider to choose an image) i 1
A pure sample of Substance S is put into an evacuated flask. The flask is then heated steadily and the temperature measured as time passes. The results are graphed below, in the middle (in green). Identical experiments are now run on Substance Y and Substance Z. Substance Y is just like S except that it has a higher heat capacity in the solid phase Cp(s). Substance Z is just like S except that it has a lower enthalpy of fusion AH. Select the graphs below, on the left and right, that show the results you expect for these new experiments. Substance Y (higher C(s)) (Drag the slider to choose an image) ¡ temperature (°C) Substance S added heat (kJ/mol) Substance Z (lower ΔΗ.) (Drag the slider to choose an image) i 1
Chemistry for Engineering Students
4th Edition
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Lawrence S. Brown, Tom Holme
Chapter9: Energy And Chemistry
Section: Chapter Questions
Problem 9.106PAE
Related questions
Question
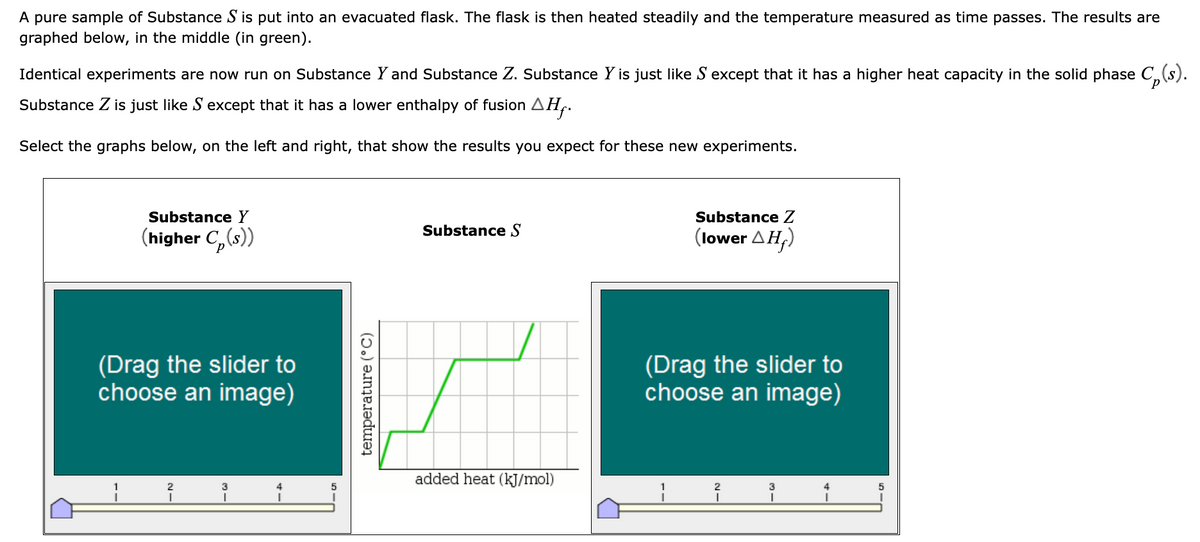
Transcribed Image Text:A pure sample of Substance S is put into an evacuated flask. The flask is then heated steadily and the temperature measured as time passes. The results are
graphed below, in the middle (in green).
Identical experiments are now run on Substance Y and Substance Z. Substance Y is just like S'except that it has a higher heat capacity in the solid phase C₂ (s).
Substance Z is just like S except that it has a lower enthalpy of fusion AH₁.
Select the graphs below, on the left and right, that show the results you expect for these new experiments.
Substance Y
(higher C(s))
(Drag the slider to
choose an image)
2
³
1
5
temperature (°C)
Substance S
added heat (kJ/mol)
Substance Z
(lower AH₂)
(Drag the slider to
choose an image)
21
3
Expert Solution
Step 1
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,