A student dissolved 1.50 g of solid Ni(NO3)2 in 30 mL distilled H2O and subjected it to a hot water bath for 5 minutes. After heating, a light green solution was produced which was then separated into six test tubes. To determine the relative stability of Ni2+ complexes, distilled H2O, 3.0 M 1,10-phenanthroline (phen), and 12 M NH3 were used as ligand sources. The observations are as follows (see the attached photo): Compute for the formation constant of [Ni(NH3)6]2+ in test tube 3 given the following equilibrium concentrations: [Ni(H2O)6 2+] = 2.5 x 10-2 M;[NH3] = 0.010 M; [Ni(NH3)6 2+] = 2.1 x 10-5 M.
A student dissolved 1.50 g of solid Ni(NO3)2 in 30 mL distilled H2O and subjected it to a hot water bath for 5 minutes. After heating, a light green solution was produced which was then separated into six test tubes. To determine the relative stability of Ni2+ complexes, distilled H2O, 3.0 M 1,10-phenanthroline (phen), and 12 M NH3 were used as ligand sources. The observations are as follows (see the attached photo): Compute for the formation constant of [Ni(NH3)6]2+ in test tube 3 given the following equilibrium concentrations: [Ni(H2O)6 2+] = 2.5 x 10-2 M;[NH3] = 0.010 M; [Ni(NH3)6 2+] = 2.1 x 10-5 M.
Chapter5: Errors In Chemical Analyses
Section: Chapter Questions
Problem 5.10QAP
Related questions
Question
100%
A student dissolved 1.50 g of solid Ni(NO3)2 in 30 mL distilled H2O and subjected it to a hot water bath for 5 minutes. After heating, a light green solution was produced which was then separated into six test tubes. To
determine the relative stability of Ni2+ complexes, distilled H2O, 3.0 M 1,10-phenanthroline (phen), and 12 M NH3 were used as ligand sources. The observations are as follows (see the attached photo):
Compute for the formation constant of [Ni(NH3)6]2+ in test tube 3 given the following equilibrium concentrations: [Ni(H2O)6 2+] = 2.5 x 10-2 M;[NH3] = 0.010 M; [Ni(NH3)6 2+] = 2.1 x 10-5 M.
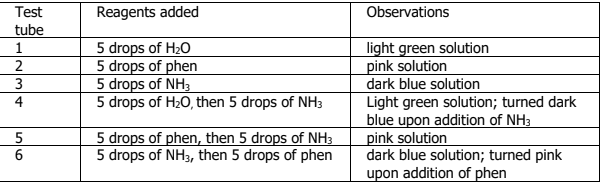
Transcribed Image Text:Test
Reagents added
Observations
tube
1
light green solution
pink solution
dark blue solution
Light green solution; turned dark
blue upon addition of NH3
pink solution
dark blue solution; turned pink
upon addition of phen
5 drops of H20
5 drops of phen
5 drops of NH3
5 drops of H20, then 5 drops of NH3
2
3
4
5 drops of phen, then 5 drops of NH3
5 drops of NH3, then 5 drops of phen
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

