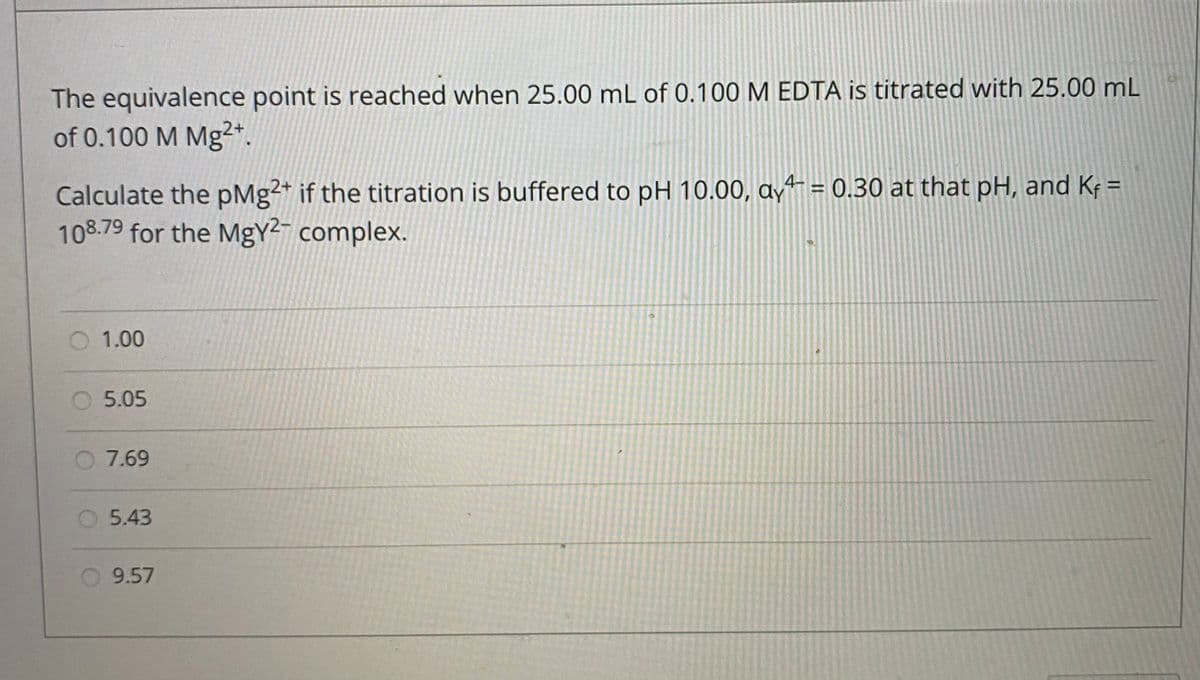
The equivalence point is reached when 25.00 mL of 0.100 M EDTA is titrated with 25.00 mL of 0.100 M Mg2*. Calculate the pMg2* if the titration is buffered to pH 10.00, ayt = 0.30 at that pH, and Kf = 108.79 for the MgY2- complex. O 1.00 5.05 O 7.69 O 5.43 O 9.57
Q: If 200 ml of 0.01 M EDTA is added to 100 ml of 0.01 M Ni solution that is buffered at PH of 10.2,…
A:
Q: A 140.0 mL sample of 0.070 M Ca²+ is titrated with 0.070 M EDTA at pH 9.00. The value of log Kf for…
A: “Since you have posted a question with multiple sub-parts, we will solve first three subparts for…
Q: 12-G. Iminodiacetic acid forms 2:1 complexes with many metal ions: CH2CO;H H2N = H3X* CH2CO3H ax2 =…
A:
Q: Explain the analogies between the titration of a metal with EDTA and the titration of a strong acid…
A: EDTA has four carboxyl groups and two amine groups that can acts as electron pair donors or Lewis…
Q: A 50.00 ml aliquot solution containing 0.524 g of magnesium sulfate (FW 120.37 in 0.500 L required…
A: Given that - Volume of magnesium sulfate = 50.00 mL Mass of magnesium sulfate in 0.500 L Solution…
Q: (ii) Consider the titration of 25.00 mL of 0.02000 M CaSO4 with 0.01000 M EDTA at pH 10.00. Write…
A: volume of EDTA = 20ml volume of CaSO4 = 25ml pH = 10
Q: 0.8153 g of a sample containing Pb(NO3)2 was taken, dissolved in water, and 40.20 mL of 0.06 M EDTA…
A: A multiple choice question based on EDTA titration, which is to be accomplished.
Q: 25.00 mL 0.01000 M Ni2+ is titrated with 0.01000 M EDTA in a solution buffered topH 5.0. Given that…
A: The formation constant can be described as the constant that is used to establish the relation…
Q: The Cr plating on a surface that measured 3.00 cm x 4.00 cm was dissolved in HCl. The pH was…
A: Given : molarity of EDTA : 0.01441M Volume of EDTA = 10.19mL Total amount of EDTA or milimole of…
Q: The Cr plating on a surface that measured 3.00 cm x 4.00 cm was dissolved in HCI. The pH was…
A:
Q: An unknown solution containing 25.00 mL of Ni2* in dilute HCI is treated with a known excess of…
A: Introduction : This question is basically based on back titration concept . Given : Molarity of…
Q: A 30-mL portion of a solution containing Ca2+ and Mg2+ was titrated with 28.19 mL of 0.213 M EDTA at…
A: Complexometric titration with EDTA is used for the determination of metal ions line calsium and…
Q: A 150.0 mL sample of 0.080 M Ca2+ is titrated with 0.080 M EDTA at pH 9.00. The value of log Kf for…
A: Given that : The volume of the Ca2+ sample = 150.0 mL Molarity of the Ca2+ sample = 0.080 M Molarity…
Q: 25.00 mL 0.01000 M Ni2+ is titrated with 0.01000 M EDTA in a solution buffered topH 5.0. Given that…
A:
Q: What is the pCu of the resulting solution if 25.00 mL of 0.400 MEDTA is added to 15.00 mL of 0.250 M…
A: This is a method of quantitative estimation of concentration of unknown solution by titrating it…
Q: What is the value of pMg for 50.0 ml of a 0.0500 M Mg*2 solution buffered at pH 10.00 and titrated…
A: Question is based on the concept of quantitative analysis. We have to calculate P value of magnesium…
Q: A solution was prepared by dissolving about 30.00m g of EDTA in approximately 1 L of water and…
A: The solution is given below -
Q: Cd²+ forms a stable complex with EDTA. Consider the titration of 30.00 ml of 0.0525 M Cd with 0.125…
A: Titration of 30.00 mL of 0.0525 M Cd with 0.125 M EDTA solution at pH 9We have to calculate the…
Q: An EDTA solution was allowed to react with Pb²⁺ to produce 0.25 M PbY²⁻, 2.67×10⁻⁸ M Pb²⁺ and an…
A: Balanced equilibrium for the reaction of EDTA(Y4-) with Pb2+ is: Pb2+(aq) + Y4-(aq) ⇌ PbY2-(aq) ;…
Q: A 120.0 mL sample of 0.040 M Ca²+ is titrated with 0.040 M EDTA at pH 9.00. The value of log Kf for…
A: COMPEXOMETRIC TITRATION Complexometric titration reactions are those reactions in which coloured…
Q: Solution made by mixing 5mL of 0.030M Co2(SO4)3 with 40mL of 0.0120M EDTA was adjusted to pH 10.…
A: Given: Concentration of Co2(SO4)3 = 0.030 M Volume of Co2(SO4)3 = 5 mL Concentration of EDTA =…
Q: For the titration of 20.0 mL of 0.0100 M EDTA with 50.0 mL of 0.0200 M MNPO4 at pH = 10.00,…
A: The given information is: Volume of EDTA = 20.0ml Molarity of EDTA= 0.010 M Volume of MnPO4 = 50.0ml…
Q: Calculate the pZn2+ for solutions prepared by adding 0.00, 5.00, 10.00, 15.00, 20.00, 25.00 and…
A: The concentration of Zn2+ can be determined as: M1V1(Zn2+)=M2V2(EDTA) pZn2+ can be determined by:…
Q: A 35.0 mL sample of 0.0200 M Cu2+ buffered at pH 8.00 is titrated with 0.0100 M EDTA. Calculate…
A: It's a multiple part question. Given information, Volume of EDTA (V1) = ? Concentration of EDTA…
Q: Determine the molar concentration of free Cu2+ in a solution prepared by combining 50.00 mL of…
A: When complex formation occurs between an analyte and titrant, the formation is measured with the…
Q: 7. Explain why Mg-EDTA complex is added to the titration mixture in the determination of calcium…
A:
Q: An EDTA solution was allowed to react with Pb²⁺ to produce 0.25 M PbY²⁻, 2.67×10⁻⁸ M Pb²⁺ and an…
A: The reaction is: Pb2++EDTA⇔PbY2-
Q: 3. A solution contains 1.694 mg of CoSo. (155.0 g/mol) per milliliter. a. Calculate the volume of…
A: a) Amount of CoSO4 = 1.694 mg/mL Calculation of mass of CoSO4: Mass=1.694 mg mL-1×25 mL=42.35 mg…
Q: 11. A solution of 0.0500M Ca²* and 0.00500M Mg* was titrated against EDTA at pH = 10 (K'r for CaY2…
A: EDTA is ethylenediaminetetraacetic acid which is an indicator. It has 2 amino and 4 carboxyl groups.…
Q: A cyanide solution with a volume of 12.99 ml was treated with 30.00 mL. of Ni solution (containing…
A:
Q: Chromel is an alloy composed of nickel, iron, and chromium. A 0.6392-g sample was dissolved and…
A:
Q: You are asked to titrate a Mn3+ solution with EDTA at pH 9.00. The overall ionic strength of the…
A: Given- Log k = 25.2k =1025.2now αEDTA4-=5.4 ×10-2×1025.2 (a).k'(conditional formation constant…
Q: A cyanide solution with a volume of 13.87 mL was treated with 24.00 mL of Ni+ solution (containing…
A: Concentration of CN- can be determined from the amount of Ni2+ used in the titration of CN-.
Q: using a buffer at pH 3.0, calculate the concentration of Ni2+ in a solution that was prepared by…
A: i)
Q: A standard solution of EDTA (0.08 M) is being used to titrate 50 ml of a 0.04 M Ca 2+ solution.…
A: Q.1: Before the addition of EDTA solution, [Ca2+] = 0.04 M pCa2+ = - log[Ca2+] = - log(0.04) =…
Q: 0.8153 g of a sample containing Pb(NO3)2 was taken, dissolved in water, and 40.20 mL of 0.06 M EDTA…
A: A numerical problem based on EDTA titration, which is to be accomplished.
Q: 4. The cadmium and lead ions in a 50.00 mL sample required 40.09 mL of a 0.005000 M EDTA for…
A: A 75.00 ml solution required 21.42 mL of EDTA after marking of Cd+2. The volume corresponds to Pb+2…
Q: . Fajan of halides end-point detection with Ag* ion are characterized by Formation of a red color of…
A: Fajan's method involves the argentometry titration in which we typically determine the amount of the…
Q: In a solution kept constant by buffering to pH= 8.0, 50.0 mL of 0.0050M Ni2+ is 0,0100 In titration…
A: The question is based on the concept of complexometric titrations. We have to calculate pNi at…
Q: Cd2* forms a stabie complex with EDTA. Consider the titration of 30.00 ml of 0.0525M Ca with 0125 M…
A: The reaction that takes place is: Cd2+ + EDTA → [CdEDTA]2− 30.00 mL of 0.0525 M Cd2+ reacts with…
Q: What is the value of pMg for 50.0 ml of a 0.0500 M Mg2 solution buffered at pH 10.00 and titrated…
A: The question is based on the concept of complexometric titration. We have to calculate P value of…
Q: The concentration of a solution of EDTA was determined by standardizing against a solution of Ca2+…
A: EDTA forms 1:1 complex with Ca2+ ions.
Q: Calculate pMn² when 50.00 mL of 0,100 0 M Mn² is titrated with 25.00 mL of 0.200 0 M EDTA. The…
A: Given: 50.00 mL of 0.1000 M Mn2+ is titrated with 25.00 mL of 0.2000 M EDTA Kf = 7.76 × 1013 α =…
Q: Chromel is an alloy composed of nickel, iron, and chromium. A 0.6418-g sample was dissolved and…
A: The 0.6418 g sample contains three metals: nickel, iron, and chromium. This total sample is…
Q: 50 mL of a solution of 0.0200 M Zn2+ will be titrated with 0.0100 M EDTA in 0.0100 M NH3 at pH…
A: given values = K1 = 1.02 x 10-2, K2= 2.14 x 10-3, K3 = 6.92 x 10-7 and K4 = 5.50 x 10-11. pH = 6.0
Q: Given the following data during the potentiometric titration of 75.00 mL unknown NaCl solution by…
A:
Q: A 15.0 mL sample of 0.0100 M Cu²+ buffered at pH 9.00 is titrated with 0.0300 M EDTA. Calculate…
A: Given: Volume of Cu2+ = 15.00 mL Concentration of Cu2+ = 0.0100 M pH=9.00 To Calculate pCu2+ for…
Q: An unknown solution containing 25.00 mL of Ni2+ in dilute HCl is treated with a known excess of…
A: Given, Ni2+=25.0 mLEDTA= 25.0 mLConcentration of EDTA =0.05382 MZn2+=0.02299 MVolume= 17.0 mL
Q: 0.8153 g of a sample containing Pb(NO3)2 was taken, dissolved in water, and 40.20 mL of 0.06 M EDTA…
A: The question is based on the concept of complexometric titrations. we have to calculate mass…
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 3 images

- The accompanying data (1.00-cm cells) were obtained for the spectrophotometric titration 10.00 mL of Pd(II) with 2.44 10-4 M Nitroso R(O. W Rollins and M. M. Oldham, Anal. chem .,1971, 43, 262, DOI: 10.1021/ac60297a026). Calculate the concentration of the Pd(II) solution, given that the ligand-to-cation ratio in the colored product is 2:125.00 mL 0.01000 M Ni2+ is titrated with 0.01000 M EDTA in a solution buffered topH 5.0. Given that the formation constant for the Ni-EDTA (NiY2–) chelate is4.2 x 1018 and the 4 value at pH 5.0 is 3.54 x 10–7. Explain briefly why EDTA is an important reagent used in complexometrictitrations.50 mL of a solution of 0.0200 M Zn2+ will be titrated with 0.0100 M EDTA in 0.0100 M NH3 at pH 6.0. Ethylenediaminetetraacetic acid (EDTA) can be considered as a tetraprotic acid (H4Y). The stepwise acid dissociation constants are: K1 = 1.02 x 10-2, K2= 2.14 x 10-3, K3 = 6.92 x 10-7 and K4 = 5.50 x 10-11. The alplia value of the un-deprotonated species in a solution buffered to a certain pH is given by the following equation: a0 = [H+]4/([H+]4 + K1[H+]3 + K1K2[H+]2 + K1 K2K3[H+] + K1 K2K3K4) Calculate the alpha value of the fully deprotonated species (Y4- ) in a solution buffered to a pH of 6.0.
- Cd²+ forms a stable complex with EDTA. Consider the titration of 30.00 ml of 0.0525 M Cd with 0.125 M EDTA at pH 9, using Eriochrome black T as the indicator. Constants you may need: log K, (Cd-EDTA) 16.5. a, @ph 9= 0.052 The pCd at equivalence point (12.6 mL) is [a]A 50.00 ml aliquot of a solution containing Ca2+ and Mg2+ was buffered at pH 10 and titrated with 0.0418 M EDTA. The endpoint volume was 44.36 ml. A second aliquot of the same mixture was made strongly basic by the addition of NaOH – this causes the Mg2+ to precipitate as Mg(OH)2. The solution was then titrated with the 0.0418 M EDTA and the endpoint volume was found to be 30.02 ml. Calculate the molar concentration of Mg.25.00 mL 0.01000 M Ni2+ is titrated with 0.01000 M EDTA in a solution buffered to pH 5.0. Given that the formation constant for the Ni-EDTA (NiY2–) chelate is 4.2 x 1018 and the 4 value at pH 5.0 is 3.54 x 10–7 An indicator (Ind) forms a metal-indicator complex with Ni2+ (NiInd), giving aconditional formation constant of 1.00 x 108 at pH 5.0. It is generally assumedthat human eyes can detect about 1 part of one color in 10 parts of another;therefore, the first discernible color change will occur when the [NiInd]:[Ind]ratio changes from 10 to about 0.1.
- An EDTA solution was allowed to react with Pb²⁺ to produce 0.25 M PbY²⁻, 2.67×10⁻⁸ M Pb²⁺ and an excess of 0.10 M at equilibrium (K = 1.1×10⁸). What will be the α₄ value under these conditions?A 50.00 ml aliquot of a solution containing Ca2+ and Mg2+ was buffered at pH 10 and titrated with 0.0499 M EDTA. The endpoint volume was 40.17 ml. A second aliquot of the same mixture was made strongly basic by the addition of NaOH – this causes the Mg2+ to precipitate as Mg(OH)2. The solution was then titrated with the 0.0499 M EDTA and the endpoint volume was found to be 34.70 ml.Consider the titration of 25.0 mL of 0.020 0 M MnSO4 with 0.010 0 M EDTA in a solution buffered to pH 6.00. Calculate pMn21 at the following volumes of added EDTA and sketch the titration curve: 0, 20.0, 40.0, 49.0, 49.9, 50.0, 50.1, 55.0, and 60.0 mL.
- 25.00 mL 0.01000 M Ni2+ is titrated with 0.01000 M EDTA in a solution buffered topH 5.0. Given that the formation constant for the Ni-EDTA (NiY2–) chelate is4.2 x 1018 and the 4 value at pH 5.0 is 3.54 x 10–7. Calculate the pNi at the equivalence point.The sulfate in a247.1 mg sample was precipitated as BasO4 by addition of 25.00 mL of 0.03992 M BaCl2. The precipitate was removed by filtration and the remaining BaCl2 consumed 36.09 mL of 0.0217 M EDTA for titration to the Camalgite endpoint. Calculate the % SO3 in the sample.What is the equivalence volume when 0.0500 M EDTA is titrated with 100.0 mL of 0.0500 M Mn+ buffered to a pH of 9.00?



