Abs at wavelength 1 ( = Abs at wavelength 2 ( = Sample nm) nm) Cr* Ni2* Unknown Set up the equation for the two wavelengths using Beer's Law and calculate the concentrations of the metal ions in your unknown using two simultaneous equations: at wavelength 1: A1 = A1lccr + Ailcni at wavelength 2: A2 = A2lccr + AzlCNi
Abs at wavelength 1 ( = Abs at wavelength 2 ( = Sample nm) nm) Cr* Ni2* Unknown Set up the equation for the two wavelengths using Beer's Law and calculate the concentrations of the metal ions in your unknown using two simultaneous equations: at wavelength 1: A1 = A1lccr + Ailcni at wavelength 2: A2 = A2lccr + AzlCNi
Principles of Instrumental Analysis
7th Edition
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Chapter12: Atomic X-ray Spectrometry
Section: Chapter Questions
Problem 12.3QAP
Related questions
Question
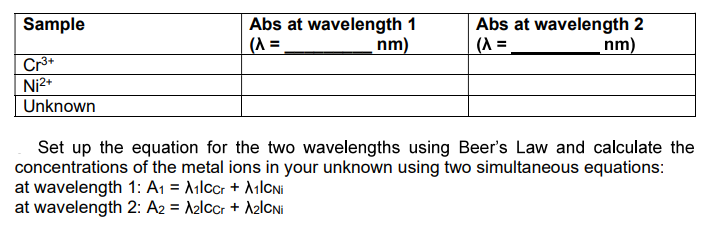
Transcribed Image Text:Abs at wavelength 1
(A =
Abs at wavelength 2
(^ =,
Sample
nm)
nm)
Cr*
Ni2+
Unknown
Set up the equation for the two wavelengths using Beer's Law and calculate the
concentrations of the metal ions in your unknown using two simultaneous equations:
at wavelength 1: A1 = Ailccr + AılCNi
at wavelength 2: A2 = A2lCcr + A2lCNi
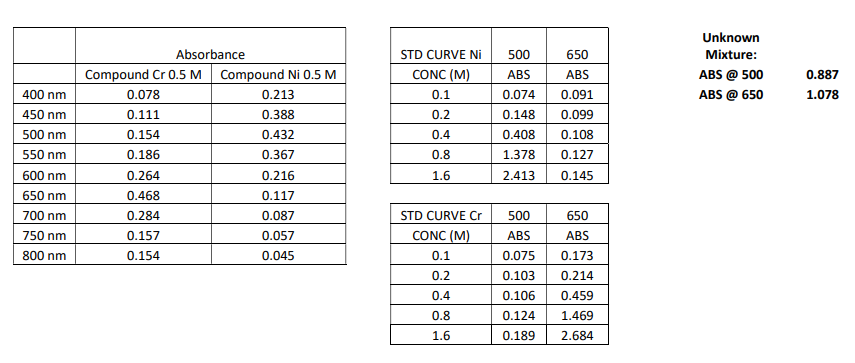
Transcribed Image Text:Unknown
Absorbance
STD CURVE Ni
500
650
Mixture:
Compound Cr 0.5 M
Compound Ni 0.5 M
CONC (M)
ABS
ABS
ABS @ 500
0.887
400 nm
0.078
0.213
0.1
0.074
0.091
ABS @ 650
1.078
450 nm
0.111
0.388
0.2
0.148
0.099
500 nm
0.154
0.432
0.4
0.408
0.108
550 nm
0.186
0.367
0.8
1.378
0.127
600 nm
0.264
0.216
1.6
2.413
0.145
650 nm
0.468
0.117
700 nm
0.284
0.087
STD CURVE Cr
500
650
750 nm
0.157
0.057
CONC (M)
ABS
ABS
800 nm
0.154
0.045
0.1
0.075
0.173
0.2
0.103
0.214
0.4
0.106
0.459
0.8
0.124
1.469
1.6
0.189
2.684
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 8 steps with 10 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning