Abs vs Time Trial 1 Ln(Abs) vs Time Trial 1 1/Abs vs Time Trial 1 0.35 0.00 9.00 20 40 60 80 100 120 140 160 180 8.00 y= 0.0297x + 2.4624 R=0.989 0.3 y=-0.0012x + 0.3231 -a.s0 7.00 R= 0.9771 0.25 y=0.0059x - 1.0624 6.00 0.2 -1.00 R0.9993 5.00 0.15 4.00 . .. -1.50 3.00 0.1 2.00 0.05 -2.00 1.00 0.00 20 40 60 80 100 120 140 160 180 -2.50 20 40 60 80 100 120 140 160 180
Abs vs Time Trial 1 Ln(Abs) vs Time Trial 1 1/Abs vs Time Trial 1 0.35 0.00 9.00 20 40 60 80 100 120 140 160 180 8.00 y= 0.0297x + 2.4624 R=0.989 0.3 y=-0.0012x + 0.3231 -a.s0 7.00 R= 0.9771 0.25 y=0.0059x - 1.0624 6.00 0.2 -1.00 R0.9993 5.00 0.15 4.00 . .. -1.50 3.00 0.1 2.00 0.05 -2.00 1.00 0.00 20 40 60 80 100 120 140 160 180 -2.50 20 40 60 80 100 120 140 160 180
Principles of Instrumental Analysis
7th Edition
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Chapter33: Automated Methods Of Analysis
Section: Chapter Questions
Problem 33.4QAP
Related questions
Question
I need help finding the rate law for each graph. The first graph is in order zero, the middle is in order first and the last is in order second.
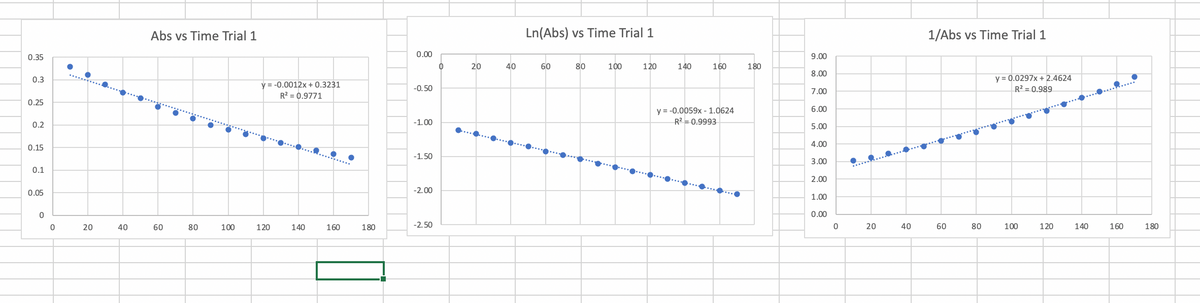
Transcribed Image Text:Abs vs Time Trial 1
Ln(Abs) vs Time Trial 1
1/Abs vs Time Trial 1
0.35
0.00
9.00
20
40
60
80
100
120
140
160
180
8.00
y = 0.0297x + 2.4624
R2 = 0.989
0.3
y = -0.0012x + 0.3231
R2 = 0.9771
-0.50
7.00
0.25
y = -0.0059x - 1.0624
R2 = 0.9993
6.00
0.2
-1.00
5.00
...
4.00
0.15
-1.50
3.00
....
0.1
2.00
0.05
-2.00
1.00
0.00
40
60
80
100
120
140
160
180
-2.50
20
40
60
80
100
120
140
160
180
20
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Recommended textbooks for you

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning


Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning
