After establishing the response factor of the instrument, the researcher collected 14.58 g of spinach, homogenized the sample, and extracted the DDT using an established method, producing a 2.75 mL solution containing an unknown amount of extracted DDT. The researcher then prepared a sample for analysis that contained 0.750 mL of the unknown DDT solution and 1.25 mL of 11.32 mg/L chloroform, which was diluted to a final volume of 25.00 mL. The sample was analyzed using GCMS, producing peak areas of 8097 and 14601 for the DDT and chloroform, respectively. Calculate the amount of DDT in the spinach sample. Express the final answer as milligrams of DDT per gram of spinach. amount of DDT: mg DDT /g spinach
After establishing the response factor of the instrument, the researcher collected 14.58 g of spinach, homogenized the sample, and extracted the DDT using an established method, producing a 2.75 mL solution containing an unknown amount of extracted DDT. The researcher then prepared a sample for analysis that contained 0.750 mL of the unknown DDT solution and 1.25 mL of 11.32 mg/L chloroform, which was diluted to a final volume of 25.00 mL. The sample was analyzed using GCMS, producing peak areas of 8097 and 14601 for the DDT and chloroform, respectively. Calculate the amount of DDT in the spinach sample. Express the final answer as milligrams of DDT per gram of spinach. amount of DDT: mg DDT /g spinach
Chapter88: Column Chromatography
Section: Chapter Questions
Problem 3P
Related questions
Question
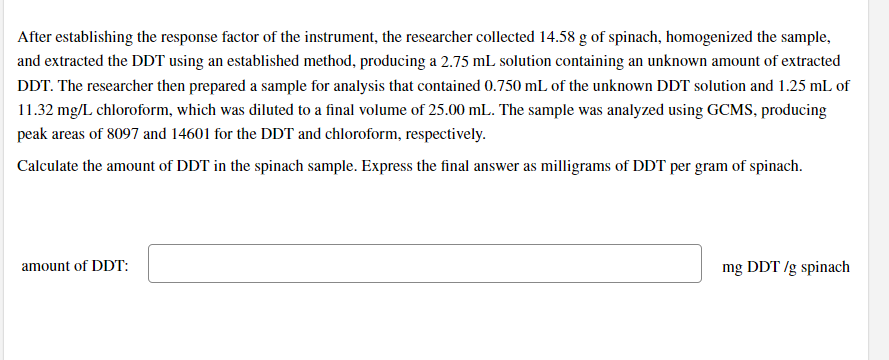
Transcribed Image Text:After establishing the response factor of the instrument, the researcher collected 14.58 g of spinach, homogenized the sample,
and extracted the DDT using an established method, producing a 2.75 mL solution containing an unknown amount of extracted
DDT. The researcher then prepared a sample for analysis that contained 0.750 mL of the unknown DDT solution and 1.25 mL of
11.32 mg/L chloroform, which was diluted to a final volume of 25.00 mL. The sample was analyzed using GCMS, producing
peak areas of 8097 and 14601 for the DDT and chloroform, respectively.
Calculate the amount of DDT in the spinach sample. Express the final answer as milligrams of DDT per gram of spinach.
amount of DDT:
mg DDT /g spinach
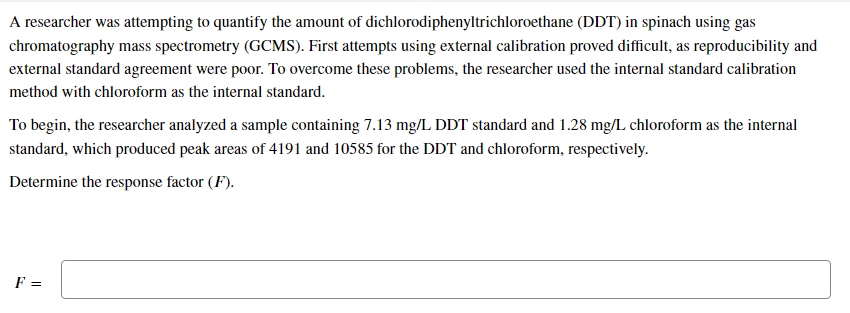
Transcribed Image Text:A researcher was attempting to quantify the amount of dichlorodiphenyltrichloroethane (DDT) in spinach using gas
chromatography mass spectrometry (GCMS). First attempts using external calibration proved difficult, as reproducibility and
external standard agreement were poor. To overcome these problems, the researcher used the internal standard calibration
method with chloroform as the internal standard.
To begin, the researcher analyzed a sample containing 7.13 mg/L DDT standard and 1.28 mg/L chloroform as the internal
standard, which produced peak areas of 4191 and 10585 for the DDT and chloroform, respectively.
Determine the response factor (F).
F =
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning


EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

