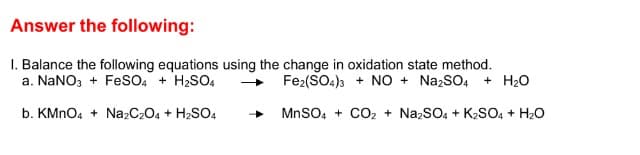
Answer the following: I. Balance the following equations using the change in oxidation state method. a. NaNO3 + FeSsO4 + H2SO, Fe2(SO4)3 + NO + NazSO4 + H20 b. KMNO, + Na2C2O4 + H2SO4 MnSO, + CO2 + NazSO, + K2SO, + H20
Q: Pourbaix diagram for the stability of water, A-F represent species position at the diagram hased on…
A: Since you have posted a question with multiple sub-parts, we will solve first three subparts for…
Q: A sample of steel weighing 2.00 g is analyzed for Cr (AW 52.0). The Cr is oxidized into chromate…
A:
Q: 2. Consider reaction-4 in the procedure: (a) How is the reaction between MnO4 and I in basic…
A:
Q: 1. Balance the following net reactions and identify the oxidizing agent and the reducing agent for…
A: Hi, as you have posted multiple questions and have not mentioned which question you want us to solve…
Q: 1. Balance the following reactions in both acidic and basic media, identify the oxidation and…
A: Please note that I have been asked to solve only part C so here I am.....
Q: A sample of steel weighing 2.00 g is analyzed for Cr (AW 52.0). The Cr is oxidized into chromate…
A:
Q: Does the cell voltage increase or decrease when 5 mL of 6 MNH3 was added to the half-cell containing…
A: Given : Concentration of NH3 added = 6 M Volume of NH3 solution added = 5 mL And the half cell is…
Q: Consider the half reactions shown below with their associated standard reduction potentials at 25 ∘C…
A: First of all we have to calculate the cell potential of the overall reaction.
Q: 2. Given the following table of standard reduction potentials for a hypothetical element X under…
A: The balanced reduction half-reactions for each of the given call notation in acidic medium(H+) are:…
Q: In which of the below reactions is metal sulfide dissolution more energetically favorable? MnS + H+…
A: The given reactions are : MnS + H+ <--> Mn2+ + HS- CuS + H+ <-->Cu2+ + HS-
Q: The reduction potentials for Au3+ and Ni2+ are: Au3+ +3e- →Au E° = +1.50 V Ni2 + 2e- Ni E° = -0.23 V…
A:
Q: uction potentials for Au3+ and Ni2+ are as follows: Au3+ + 3e Au, E = +1.50 V Ni2+ + 2e Ni, E =…
A:
Q: A solution containing Pt* is electrolyzed with a current of 5.80 A. How long will it take to plate…
A: Moles of Pt4+ to be electrolyzed: = 99% × (0.20 L) × 0.025 M= 0.99 × (0.20) × 0.025 mol= 0.00495…
Q: 2The reductant in Walden reductor, is a. any first group element O b. metallic silver OC KMNO4 O d.…
A:
Q: Describe how you would prepare 1litre of 150ppm Cu2+ using Cu metal
A: ppm is defined as the parts of solute or mass of solute present per million parts of solution Hence…
Q: Iron corrodes to produce rust, Fe2O3, but other corrosionproducts that can form are Fe(O)(OH), iron…
A:
Q: vo; 1.000 →VO²+ 0337 √³+ 5 ) 172+ -0.255 -1.13 V acid solution
A:
Q: A sample of steel weighing 2.00 g is analyzed for Cr (AW 52.0). The Cr is oxidized into chromate…
A: Solution -
Q: For (c), why doesn't SO42- oxidize Na+ when the reduction potential of Na+ (-2.71V) is lower than…
A: If electrons get donated by any species/neutral and that species gets converted into +ve ion then…
Q: A sample of pyrolusite weighs 0.5000 g. To this is added 0.6674 g of As2O3 and dilute acid. After…
A: NOTE
Q: I need the answer as soon as possible
A:
Q: The pyrite (iron sulfide) roasting process is carried out following the next reaction [FeS2 (s) +…
A:
Q: Calculate the potential of a platinum electrode immersed in a solution that is (a) 0.0613 M in…
A: “Since you have posted a question with multiple sub-parts, we will solve first three sub-parts for…
Q: a) Chlorine is disproportionate in basic medium. And in an acid medium? Write both reactions and…
A: Since you have posted 2 questions, we will solve first question for you. Please repost other…
Q: Calculate the time, in minutes, needed to plate 17.697 g of metal M at 43 Amps. The molar mass of…
A: Consider the given reaction as; M6+ + 6e- → M
Q: chloride was passed into potassium iodide solution where it liberated iodine: Cl2 + KI → KCl + I2…
A: Calculate from given data
Q: A cell consisting of a saturated calomel electrode and a lead ion electrode developed a potential of…
A:
Q: Calculate the theoretical potential at 25°C needed to initiate the deposition of copper from a…
A:
Q: Cobalt(II) ions (Co2*(aq)) react with 6 equivalents of a ligand (L) to from the complex cation…
A: Reaction of Co2+ with L occurs as: Co2+(aq)+6L↔CoL62+
Q: 13. Redox active species can be quantitated using electrogravimetric analysis. d. For a colored…
A: Electrogravimetric analysis used to separate ,quantify metal ion.In this process analyte solution…
Q: Find the normality of the solution containing 4.63 g/l k2Cr207 (Fwt 294.19) if cr+6 is reduced to…
A: Here the chromium is in +6 oxidation state it is reduced to +6 we need to calculate the normality
Q: A solution containing 0.402 49 g of CoCl2 ? xH2O (a solid with an unknown number of waters of…
A: Moles: It is defined as the measurement of a substance that contains 6.022×1023 number of particles…
Q: diagram for Thallium Tl3+ /Tl+ = 1.25 V Tl+ /Tl = -0.34 V a) Indicate which is…
A: 1)Due to inert pair effect thallium have +1 oxidation state Or ∆G=-nRTE°cell Reaction will go for…
Q: Calculate the concentration of 12 solution saturated with Agl Eº = that gives a potential of a…
A:
Q: Which of the following species can oxidize both Mn(s) and Cd(s) but not Pb(s)? e° of Cd|Cd2+ = -0.40…
A: Given information, E° of Cd|Cd2+ = -0.40 V E° of Mn|Mn2+ = -1.18 V E° of Pb|Pb2+ = -0.13 V
Q: A sample of pyrolusite weighing 0.6000 g is dissolved in a solution containing 5.00 mL of 6.00 N…
A: Given that 1 mL of KMnO4 oxidise 0.03058 g of FeSO4.7H2O…
Q: The following potential diagram summarizes the results of electrochemical studies of the aqueous…
A:
Q: Given that standard reduction potentials at 298.15 K are E° Mg*2 /Mg = -2.37 V and E° Sn2*/Sn =…
A:
Q: 4. A 0.200-g sample of pyrolusite is analyzed for manganese content as follows: 50.0 ml of 0.100 M…
A: Sample of pyrolusite analyzed = 0.200 g Molarity of Ferrous ammonium sulphate=0.100 M Volume of…
Q: A dilute solution of CuSO4 was electrolyzed using Pt- electrodes. The amount of Cu in the anodic…
A: Given that: amount of Cu in the anodic solution before electrolysis = 0.6350 g amount of Cu in…
Q: Calculate the time, in minutes, needed to plate 17.697 g of metal M at 43 Amps. The molar mass of…
A: this question is based on faraday second law: the mass of different ions liberated at the electrode…
Q: Which of the following species can oxidize both Mn(s) and Cd(s) but not Pb(s)? e° of Cd|Cd²+ = -0.40…
A: As Ni has reduction potential greater than that of Mn and Cd, so it can oxidise both Mn and cd. But…
Q: A solution containing Pt“t is electrolyzed with a current of 4.30 A. How long will it take to plate…
A: This above question is solved with the help of Faraday's first law.. 1 Faraday = 96500 coulomb
Q: Consider the reaction between dissolved Fe²* and elemental sulfur (S°) to form hematite (Fe2O3) and…
A: Given reaction : Fe2++So→Fe2O3+HS- Reduction is defined as the gain of electron while the oxidation…
Q: Give the cell diagram for the reaction below using | and || when applicable. Cd + 2 Fe3+ ---->Cd2+ +…
A: Applying basic representation of cell.
Step by step
Solved in 3 steps

- (a) What is the percentage of MnO2 in a pyrolusite ore if a sample weighing 0.4000g is treated with 0.6000g of pure H2C2O4•2H2O and dilute H2SO4 and after reduction has taken place (MnO2 + H2C2O4 + 2H+→ Mn2++ 2CO2 + 2H2O), the excess oxalic acid requires 26.26ml of 0.1000N KMnO4 for titration? (b) If pure As2O3 were used instead of oxalic acid, how many grams would be required in order for the other numerical data to remain the same?(a) What is the percentage of MnO2 in a pyrolusite ore if a sample weighing 0.4000 g is treated with a 0.6000 g of pure H2C2O4.2H20 and dilute H2SO4 and after reduction has taken place (MnO2 + H2C2O4 + 2H+ Mn2+ + 2CO2 + 2H2O), the excess oxalic acid requires 26.26 mL of 0.1000 N KMNO4 for titration? (b) If pure As2O3 were used instead of oxalic acid, how many grams would be required in order for the other numerical data to remain the same?Calculate the time, in minutes, needed to plate 17.697 g of metal M at 43 Amps. The molar mass of metal M is 57.915 g/mol. M6+ + 6 e– → M Recall that, C = A*s, and Faraday’s constant, F, is 96,485 C/mole of electrons.
- A 1.000 g sample containing chlorides, iodides and inert materials was treated with dilute nitric acid followed by AgNO3. A precipitate of AgCl (143.32) and AgI (234.77) was produced and weighs 0.9238 g. On heating in a current of Cl2, the AgI is converted to AgCl, and the resulting product weighs 0.7238 g. Find the percentage of a) NaI (149.89) and b) NaCl (58.44) in the sampleDescribe how you would prepare 1litre of 150ppm Cu2+ using Cu metalIn which of the below reactions is metal sulfide dissolution more energetically favorable? MnS + H+ <--> Mn2+ + HS- CuS + H+ <-->Cu2+ + HS-
- 35.A sample of pyrolusite weighs 0.5000 g. To this is added 0.6674 g of As2O3 and dilute acid. After solvent action has ceased, the excess three-valent arsenic is titrated with 45.00 mL of 0.1000 N KMnO4. Calculate the oxidizing power of the pyrolusite in terms of percemtage MnO2. 36. A solution of Iodinebia such concentration that 20.0 mL are required to titrate the antimony in a 0.100 g sample containing 84.93% Sb2S3(339.7 g/mol). What is the value of 1.00 mL of this Iodine in terms of grams sulfue in tiration?A concentration cell was made using a silver metal electrode in a saturated solution of AgCl for the anode half cell, and a silver metal electrode in 1.00 M Ag⁺ for the cathode half cell. E for the cell was 0.288 V at 25°C. Calculate the Ksp for AgCl. (Note: For purposes of this exercise, carry out the calculation to three significant figures.)A sample of pyrolusite weighing 0.6000 g is dissolved in a solution containing 5.00 mL of 6.00 N H2SO4 and 0.900 g of H2C2O4 .2H2O. The excess oxalate then requires 24.00 mL of KMnO4 solution for titration. If each mL of the KMnO4 will oxidize the Fe(II) in 0.03058 g FeSO4 .7H2O, what is the oxidizing power of the sample in terms of MnO2?
- In order to draw Pourbaix Diagram for a metal, all possible electrochemical and chemical reactions have to be known and with the application of Nernst equation or solubility product constant, the E-pH diagram can be constructed. We have looked at the diagram for Fe. While the Pourbaix diagram of Fe is quite complex due to its two oxidation states +2 and +3, construct a simplified Pourbaix diagram for Fe considering only the following three reactions. Note: CFe+2 and CFe+3 can be taken as 10-6 M Fe ----- Fe+2 + 2e- 2Fe+2 + 3H2O ----- Fe2O3 + 6H+ + 2e- 2Fe+3 + 3H2O ----- Fe2O3 + 6H+A sample of pyrolusite weighing 0.6000 g is dissolved in a solution containing 5.00mL of 6.00 N H2SO4 and 0.900 g of H2C2O4.2H2O. The excess oxalate then requires24.00 mL of KMnO4 solution for titration. If each mL of the KMnO4 will oxidize the Fe(II)in 0.03058 g FeSO4.7H2O, what is the oxidizing power of the sample in terms of MnO2?84.33%9 The pyrite (iron sulfide) roasting process is carried out following the next reaction [FeS2 (s) + O2 (g)]25°C → [Fe2O3 (s) + SO2(g)] 900 °C a) Determine the enthalpy balance for the reaction as stated in the reaction shown b) Determine the enthalpy balance using air with 10% excess and preheated to 500°C

